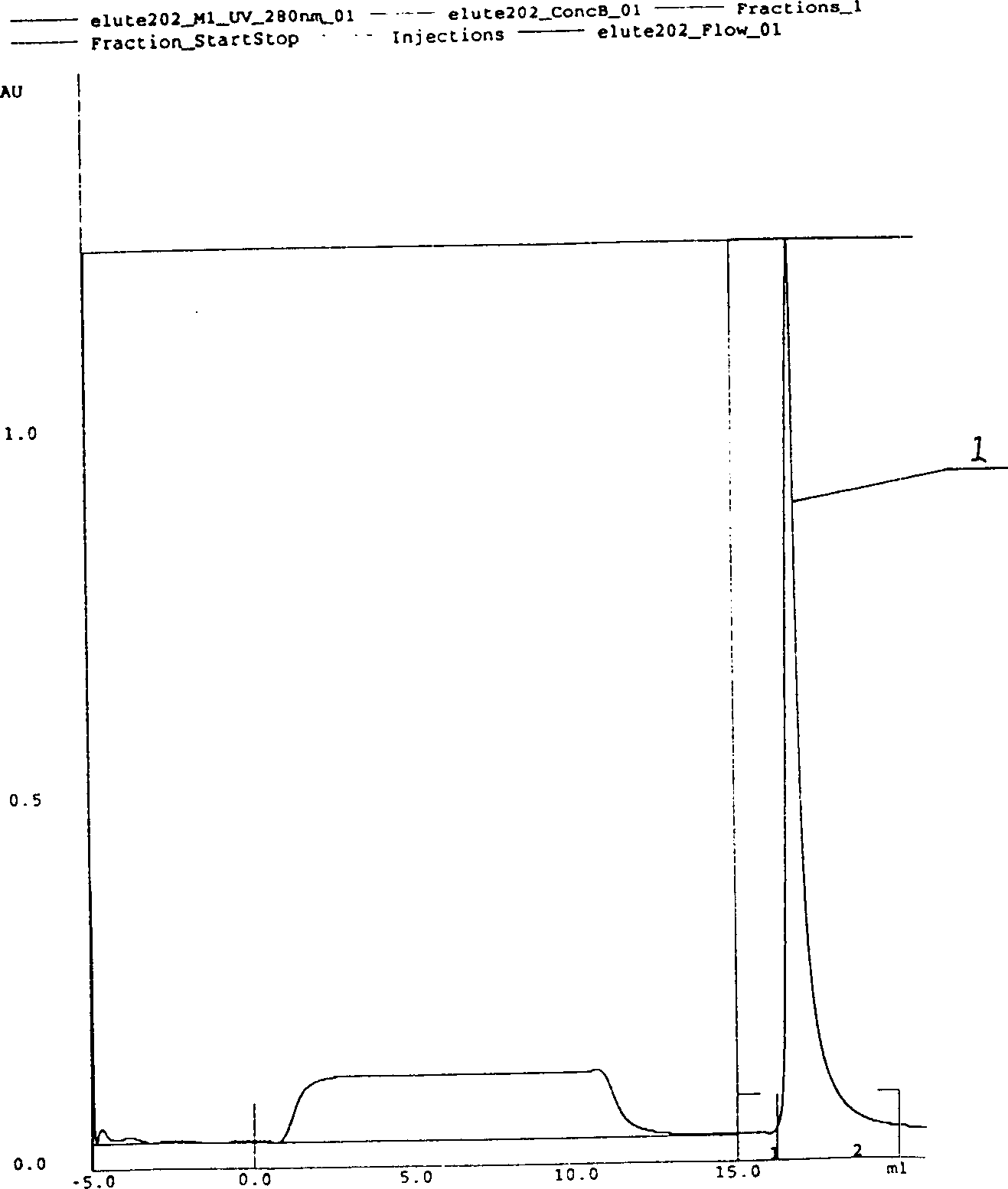
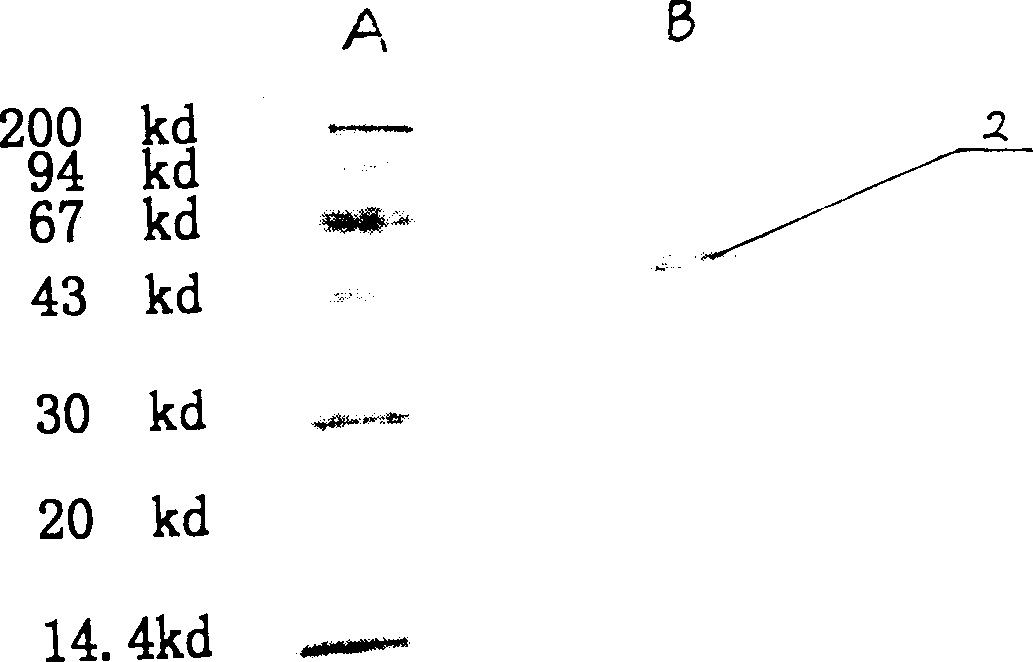
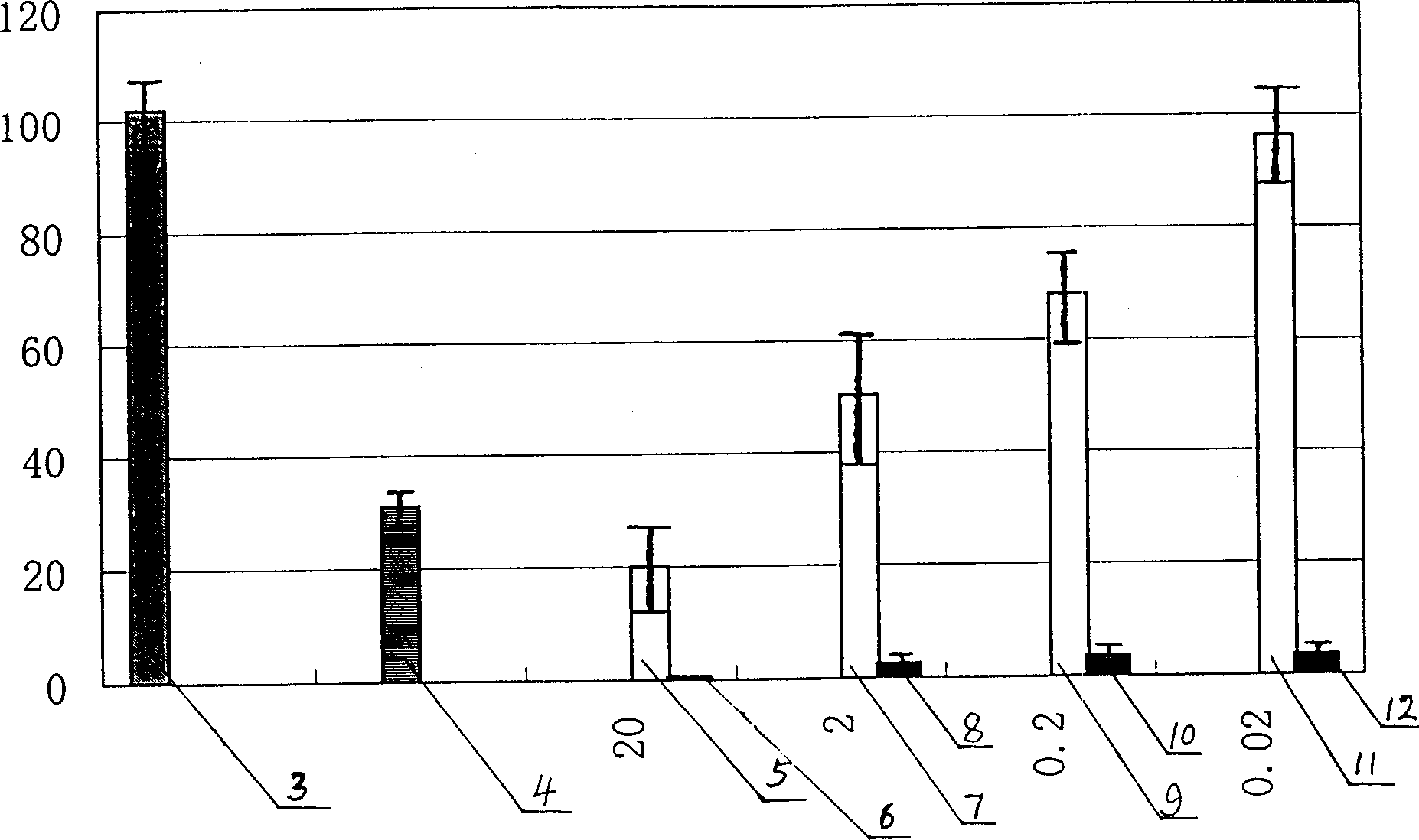
Process high effectively producing BPI-Fc recombinant protein
A recombinant protein, bpi-fc technology, applied in the field of genetic engineering, can solve the problems of influence, large molecules, and low expression of eukaryotic expression systems, achieve broad application prospects, and enhance the effect of killing G-bacteria.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0022] 1. Cloning of BPI functional fragment gene and Fcγ1 fragment gene
[0023] 1. Cloning of BPI functional fragment gene: extract mRNA from promyelocytic leukemia HL-6 cell line (ATCC CCL240), use designed and synthesized human BPI gene primers (P1 5'CAGAATTCATGGTCAACCCTGGCGTCGTG 3' and P2 5'GCAAGCTTCTATTTTGGTCATTACTGGCAG 3' ) for RT-PCR amplification (reverse transcription at 48°C for 45 minutes, followed by 35 cycles of PCR amplification, with cycle parameters of denaturation at 94°C for 30 seconds, renaturation at 53.5°C for 40 seconds, and extension at 72°C for 45 seconds), to obtain an approximately 620bp BPI gene fragment BPI 620 ; Digest BPI with EcoR I / Hind III 620 Get the BPI gene fragment BPI of about 200bp 200 (EcoR I / EcoR I cohesive end) and the BPI gene fragment BPI of about 420bp 420 (EcoR I / HindIII cohesive end); According to the molecular cloning operation method [Sambrook J.et al.Molecular cloning:laboratory manual, second edition, 1989, Cold Spring Har...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com