Application of plasma exosome biomarker in screening or curative effect prediction of gastric cancer neoadjuvant chemotherapy sensitive population
A biomarker, chemotherapy-sensitive technology, applied in the field of biomarkers, can solve the problems of inability to directly respond to tumor pathological changes, large patient damage, and difficult to achieve, and achieve the effect of promoting treatment strategies, high sensitivity, and convenient use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1 Screening of exosomal RNAs related to the efficacy of neoadjuvant chemotherapy
[0065] 1. Include objects
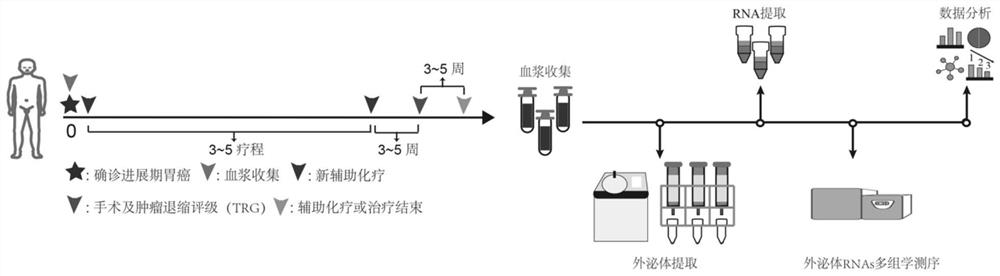
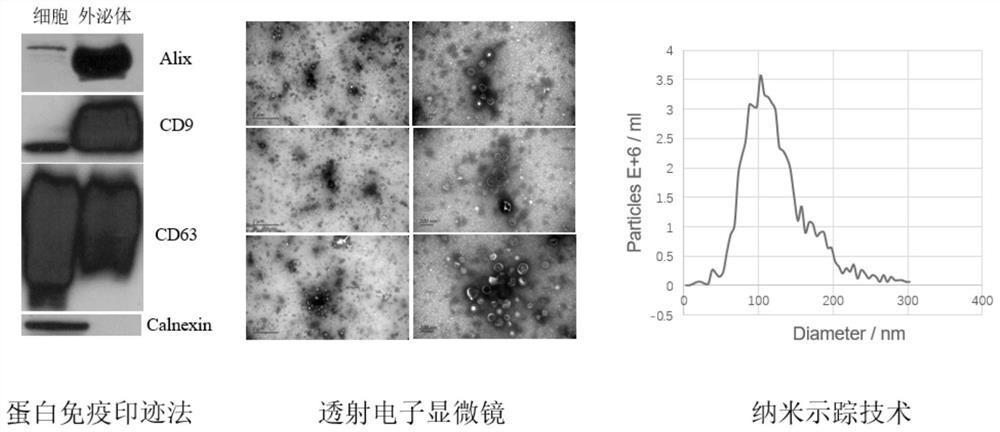
[0066] We randomly selected 10 healthy volunteers and 20 patients with advanced gastric cancer (AGC) as the included subjects. The healthy volunteers were from Peking University First Hospital, and the gastric cancer patients were from the First Ward of the Gastrointestinal Cancer Center of Peking University Cancer Hospital. Both groups were 90% male, with a male-to-female ratio of 9:1; the mean age was 57 years, and the age range was 43-73 years. All 20 AGC patients received neoadjuvant chemotherapy with SOX (oxaliplatin-seggio) or XELOX (oxaliplatin-capecitabine) regimens (these two regimens are Chinese Society of Clinical Oncology (CSCO) guidelines). (2021 version) recommended regimen), the chemotherapy regimen is: oxaliplatin (130mg / m 2 ) administered intravenously on the first day, Sigiol (40-60 mg / m 2 ) or capecitabine (1000mg / m 2 ) was orall...
Embodiment 2
[0082] Example 2. Validation of exosomal RNA biomarkers associated with neoadjuvant chemotherapy efficacy
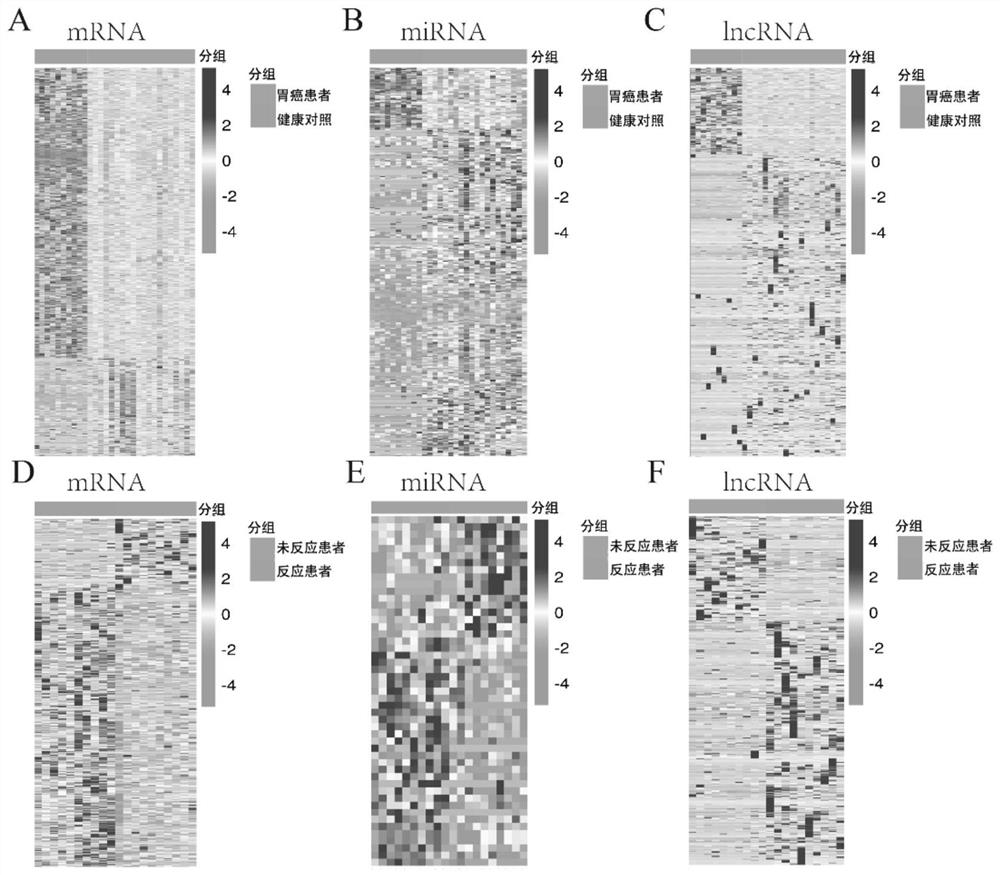
[0083]In order to further verify the predictive efficacy of exosomal RNAs, we comprehensively screened 18 exosomal RNAs with high differential multiples (specifically: 9 exosomal miRNAs (miR-27a-3p, miR-335-5p, -454-3p, miR-152-3p, miR-181a-5p, miR-1843, let-7i-5p, miR-130a-3p and miR-1307-3p), 5 exosomal mRNAs (LZIC, KMT2E , DAPP1, DAB2, and SRSF6) and 4 lncRNAs (lncPTMA-209, lncRMRP-202, lncRPS24-210, and lncFTH1-211)), using real-time quantitative PCR (qRT-PCR) in new independent 43 advanced gastric cancer patients validated in a patient cohort. The new 43 patients were also patients with advanced gastric cancer and received neoadjuvant chemotherapy with SOX or XELOX regimen and D2 gastric cancer resection, of which 19 patients had good response and 24 patients had poor response. The treatment method, curative effect standard, plasma collection method, exosome, and ...
Embodiment 3
[0093] Example 3 Construction and validation of exosomal RNA model related to the efficacy of neoadjuvant chemotherapy
[0094] To further explore better evaluation methods, we evaluated 8 exosomal RNAs (including 4 exosomal miRNAs: let-7i-5p, miR-1307-3p, miR-181a-5p and miR-181a) by Z-score method. -1843; 2 exosomal mRNAs: LZIC and SRSF6; and 2 exosomal lncRNAs: lncFTH1-211 and lncPTMA-209) were normalized for the expression of patients in the sequencing group and the validation group, and then multi-indicator Construction of the model.
[0095] Using the method of logistic regression, we firstly constructed two-molecular model group, three-molecular model group and four-molecular model group for the above 8 exosomes. There are 3 bimolecular models, 6 trimolecular models, and 2 tetramolecular models with values greater than 0.80 (Table 4, Figure 5-7 ), and their AUCs in the training set were all greater than 0.90, indicating high specificity, sensitivity and stability...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com