Preparation process of 1-mercaptomethyl cyclopropyl acetic acid
A technology of mercaptomethylcyclopropylacetic acid and phenyl, which is applied in the field of preparation technology of 1-mercaptomethylcyclopropylacetic acid, can solve separation difficulties, lower raw material utilization rate, and low ring-opening yield of cyclic sulfides By-products and other problems, to achieve the effect of simplifying the preparation process steps, low cost, and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
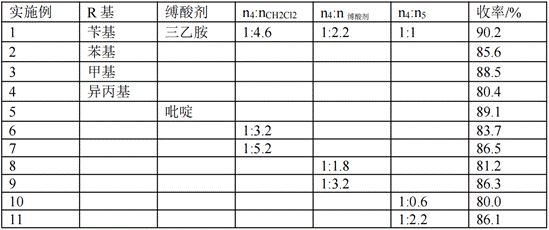
[0043] Example 1 R is benzyl, and acid binding agent is triethylamine
Embodiment 1-1
[0044] Example 1-1 Preparation of compound 3a
[0045] The chemical formula of the reaction is:
[0046] ,
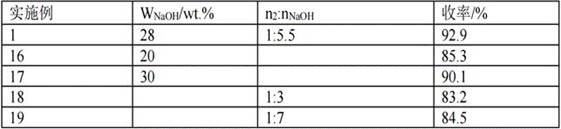
[0047] The compound 4 (11.1 g, 0.1 mol) / dichloromethane (29.5 mL, 0.46 mol) solution was cooled to 0 °C, 5a (i.e. p-toluenesulfonyl chloride, 19.1 g, 0.1 mol), triethylamine (30.5 mL, 0.1 mol) were added. 0.22mol), stir overnight, add water to dilute and separate the organic layer, extract the mixture three times with dichloromethane (50 mL each time), wash the organic matter with saturated sodium chloride (5 mL), and dry the organic matter with anhydrous magnesium sulfate. The organic extract was concentrated under reduced pressure, and the product was purified by recrystallization to obtain compound 3a. The yield in this step was 90.2%.
Embodiment 1-2
[0048] Example 1-2 Preparation of Compound 2
[0049] The chemical formula of the reaction is:
[0050] ,
[0051] A solution of thiourea (22.8 g, 0.3 mol) in 250 ml of absolute ethanol was prepared, compound 3a (0.1 mol) was added to it, refluxed for 30 h, cooled to room temperature, adjusted to pH 5 with 5N HCl, and the solvent was removed in vacuo at room temperature, Extracted twice with 80 ml of dichloromethane, washed the organic layer with saturated sodium chloride, separated and dried with anhydrous sodium sulfate, and distilled under reduced pressure to obtain compound 2. The yield in this step was 89.5%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap