Optimized GIP peptide analogs
A technology of analogs and insulin-stimulating peptides, applied in the field of peptide analogs, can solve problems such as inappropriate drug administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
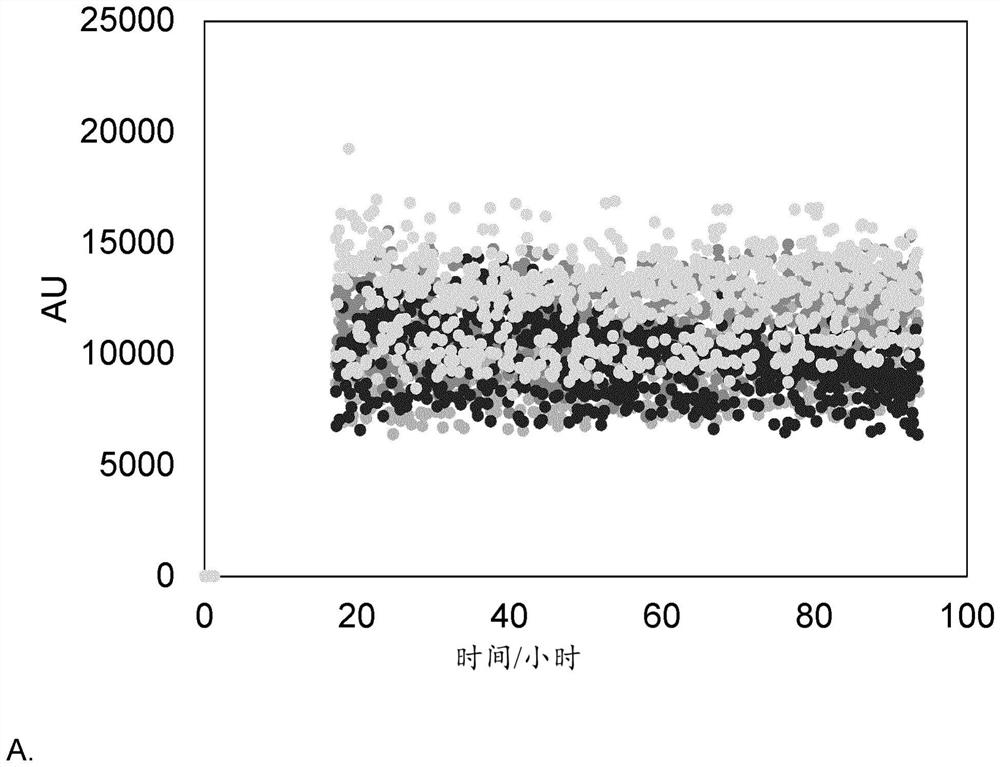
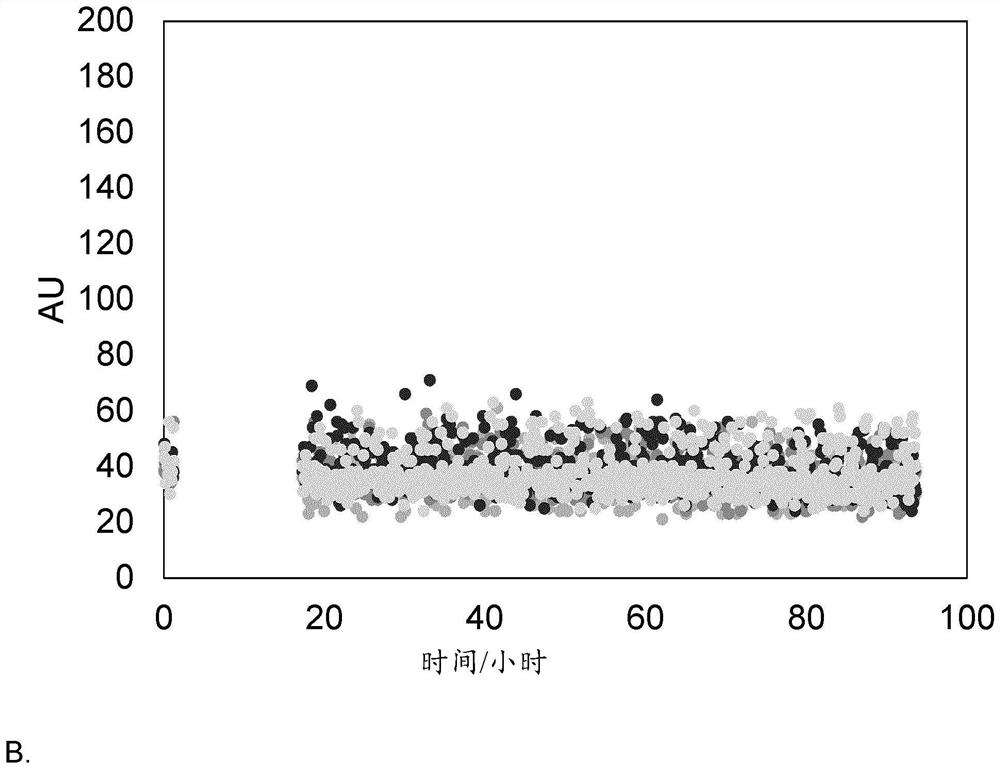
[0672] The embodiments of the present invention support the following conclusions:
[0673] 1) GIP peptide analogs comprising substitutions A13Aib and / or N24E according to embodiments of the present disclosure have increased solubility and / or stability, such as physical stability.
[0674] 2) Individual amino acid substitutions at certain positions, such as Aib at position 13, lead to improved antagonism of GIP receptors.
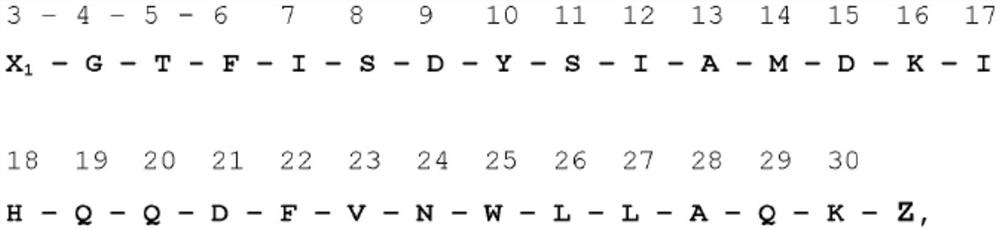
[0675] 3) Several acylation sites show great potential for GIP(3-30)+Z with substitutions A13Aib and / or N24E, such as position 18.
[0676] Materials and methods
[0677] WO 2016 / 034186 discloses the production and action of the GIP(3-30) peptide itself.
[0678] Material
[0679] Human GIP (1-42) was purchased from Phoenix Pharmaceuticals Inc., while the remaining GIP peptide analogs were synthesized by Caslo TM (Lingby, Denmark) and Almac Group (Craigavon, UK), Peptides & Elephants GmbH (Henningsdorf, Germany) and WuXi AppTec, China. The cDNA for the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com