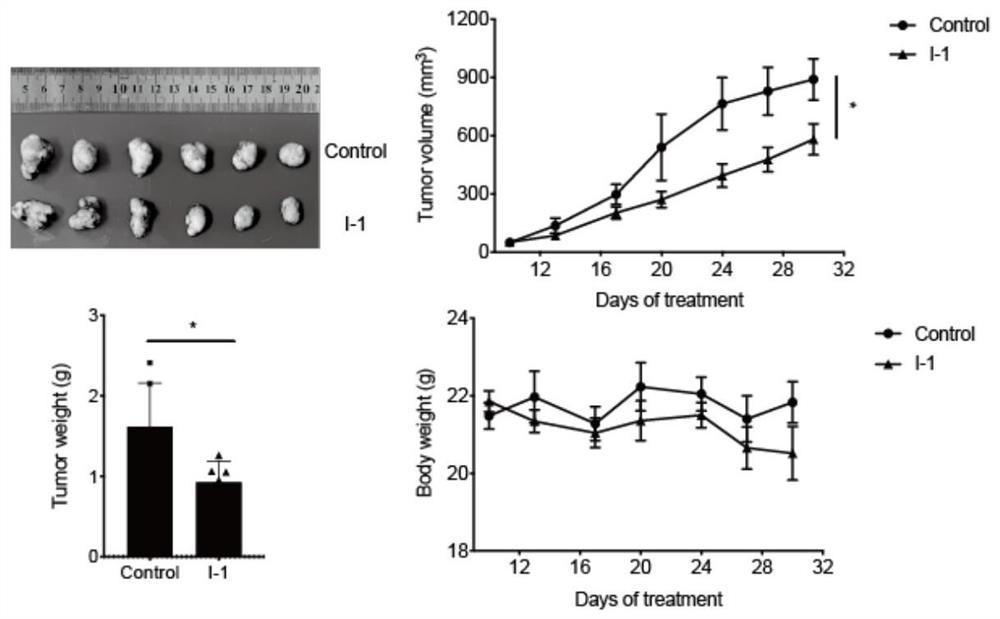
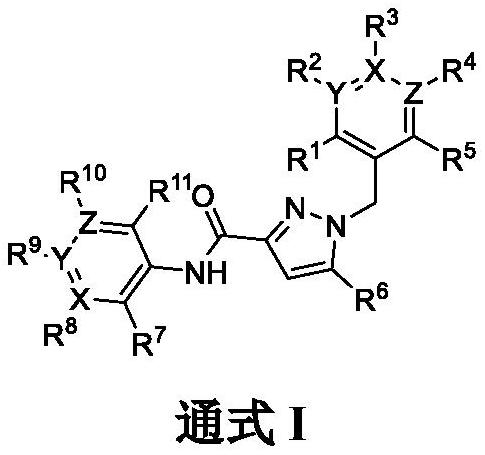
Pyrazole carboxamide derivative and preparation method and application thereof
A technology of pyrazole carboxamide and derivatives, applied in the field of chemical medicine, can solve the problems of high toxicity, poor effect, non-single target point and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1. Preparation of 1-(3-bromobenzyl)-5-methyl-1H-pyrazole-3-carboxylic acid ethyl ester (intermediate 1a)
[0045]
[0046] 300 mg (2.0 mmol) of raw material ethyl 3-methylpyrazole-5-carboxylate and 220 mg (4.0 mmol) of potassium hydroxide were placed in 5 ml of anhydrous tetrahydrofuran (THF), and 750 mg of m-bromobenzyl bromide (3.0 mg) was added dropwise under reflux. mmol) in THF, after monitoring the reaction by TLC, the reaction solution was cooled to room temperature, poured into a separatory funnel, extracted with ethyl acetate and saturated brine for 2-3 times, the organic phase was collected and washed with anhydrous Na 2 SO 4 It was dried, filtered, concentrated and purified by column chromatography, eluted with petroleum ether / ethyl acetate (10:1), to obtain 478 mg of a pale yellow oil with a yield of 74%. 1 H NMR (400MHz, DMSO-d 6 )δ7.48-7.46(m,1H),7.31-7.29(m,2H),7.11-7.09(m,1H),6.59(s,1H),5.42(m,2H),4.24(q,J= 7.1Hz, 2H), 2.24(s, 3H), 1.26(t, J=...
Embodiment 2
[0047] Example 2. Preparation of ethyl 1-(3-bromobenzyl)-1H-pyrazole-3-carboxylate (intermediate 1b)
[0048]
[0049] 280 mg (2.0 mmol) of raw material 3-ethoxycarbonylpyrazole and 220 mg (4.0 mmol) of potassium hydroxide were placed in 5 ml of anhydrous tetrahydrofuran (THF), and a solution of 750 mg (3.0 mmol) of m-bromobenzyl bromide in THF was added dropwise under reflux. , after monitoring the reaction by TLC, the reaction solution was cooled to room temperature, poured into a separatory funnel, extracted with ethyl acetate and saturated brine for 2-3 times, the organic phase was collected and washed with anhydrous Na 2 SO 4 It was dried, filtered, concentrated and purified by column chromatography, eluting with petroleum ether / ethyl acetate (10:1), to obtain 462 mg of a pale white solid with a yield of 75%. 1 H NMR (400MHz, DMSO-d 6 )δ7.43-7.41(m,2H),7.36(d,J=7.5Hz,1H),7.22-7.19(m,2H),6.82(d,J=7.5Hz,1H),5.38(m,2H) ), 4.39(q, J=7.1Hz, 2H), 1.39-1.32(m, 3H).
Embodiment 3
[0050] Example 3. Preparation of 1-(3-bromobenzyl)-5-phenyl-1H-pyrazole-3-carboxylic acid ethyl ester (intermediate 1c)
[0051]
[0052] The raw material 5-phenyl-1H-pyrazole-3-carboxylic acid ethyl ester 432 mg (2.0 mmol) and potassium hydroxide 220 mg (4.0 mmol) were placed in 5 ml of anhydrous tetrahydrofuran (THF), and m-benzyl bromide was added dropwise under reflux. A solution of bromine 750 mg (3.0 mmol) in THF, after monitoring the completion of the reaction by TLC, the reaction solution was cooled to room temperature, poured into a separatory funnel, extracted with ethyl acetate and saturated brine for 2-3 times, the organic phase was collected and washed with anhydrous Na 2 SO 4 It was dried, filtered, concentrated and purified by column chromatography, eluted with petroleum ether / ethyl acetate (10:1), to obtain 500 mg of pale yellow oil with a yield of 65%. 1 H NMR (400MHz, DMSO-d 6 )δ7.50(s, 1H), 7.42-7.23(m, 8H), 7.11(s, 1H), 5.40(m, 2H), 4.39(q, J=7.1Hz, 2H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com