Method for producing benzanthrone by using primary battery technology
A technology of benzoxantrone and primary battery, which is applied in the field of primary battery technology to produce benzoxantrone, which can solve the problems of low utilization rate of raw materials and difficult recovery of metal ions, etc., and achieve the effects of high utilization rate of raw materials, avoiding pollution, and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
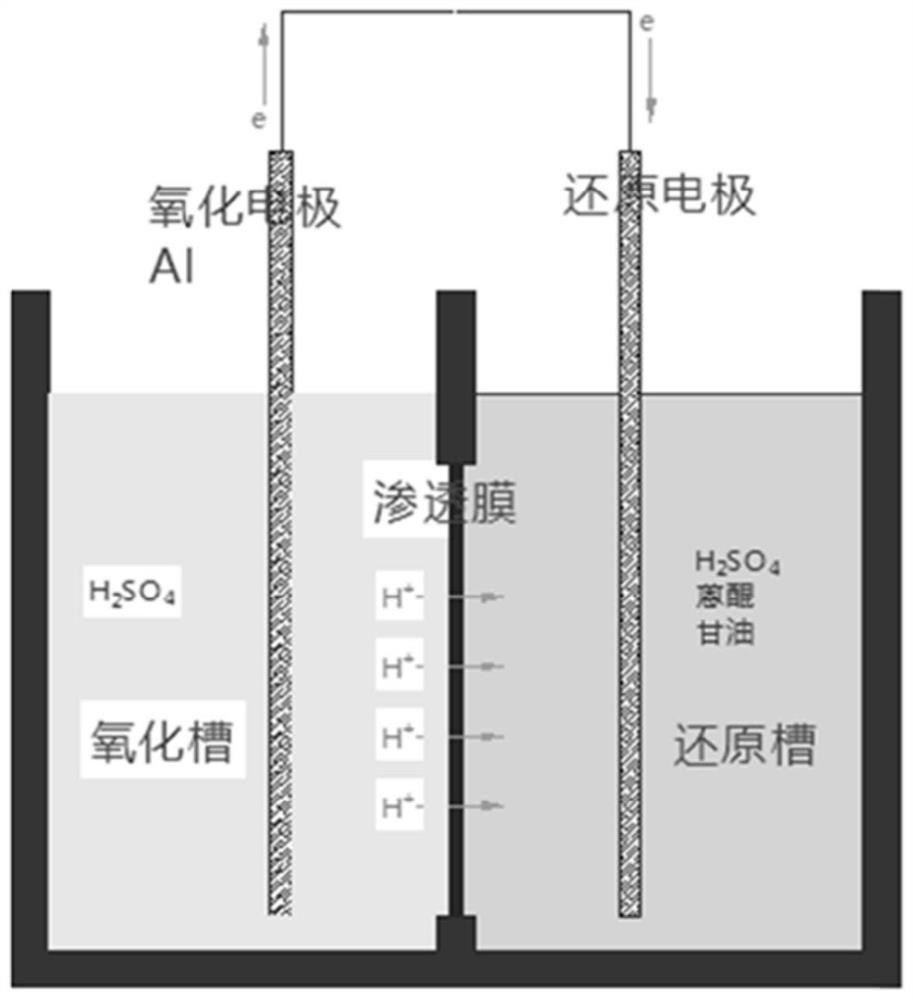
[0036] The reaction takes place in a galvanic device consisting of a cathode cell, an anode cell, and a permeable membrane (see figure 1 ) in. Add 150mL of 87% sulfuric acid, 20g of anthraquinone, and 14mL of glycerin to the cathode tank, surround the tank wall with 316L stainless steel mesh as the positive electrode of the original battery, and anthraquinone will undergo reduction reaction in it, and the liquid level of the anode tank and the cathode tank will be equal. 87% of sulfuric acid, add an aluminum plate as the anode, connect the anode and the cathode directly with a wire, and connect the ammeter in series to measure the current generated by the electrochemical reaction. When the temperature is raised to 80°C, the initial current is greater than 190mA, and the reaction solution is bright yellow. As the reaction progressed, the color of the reaction solution became darker. After stirring for 10 hours, 34% of the anthraquinone remained, and the current was 120mA-140mA....
Embodiment 2
[0039] The reaction takes place in a galvanic device consisting of a cathode cell, an anode cell, and a permeable membrane (see figure 1 ) in. Add 150mL of 90% sulfuric acid and 20g of anthraquinone to the cathode tank, surround the tank wall with a 316L net as the positive electrode of the original battery, and anthraquinone will undergo a reduction reaction therein, add 87% of the sulfuric acid in the anode tank to the level of the cathode tank, Add an aluminum plate as the anode, connect the anode and the cathode directly with a wire, and connect an ammeter in series to measure the current generated by the electrochemical reaction. When the temperature is raised to 80°C, the initial current is 170mA-190mA, and the reaction solution is bright yellow. The color of the reaction solution deepened, and after stirring for 7 hours, 38% of the anthraquinone remained, and the current was 130mA-150mA.
[0040] Add 7.7mL of glycerol to the above reaction solution, heat and stir for 4...
Embodiment 3
[0043] The reaction takes place in a galvanic device consisting of a cathode cell, an anode cell, and a permeable membrane (see figure 1 ) in. Add 150mL of 90% sulfuric acid and 20g of anthraquinone to the cathode tank, surround the tank wall with a 316L stainless steel mesh as the positive electrode of the primary battery, in which anthraquinone undergoes a reduction reaction, add 87% sulfuric acid to the anode tank and the level of the cathode tank , add an aluminum plate as the anode, connect the anode and the cathode directly with a wire, and connect the ammeter in series to measure the current generated by the electrochemical reaction. When the temperature is raised to 80°C, the initial current is 170mA-190mA, and the reaction solution is bright yellow. The color of the reaction solution was deepened, and the anthraquinone remained at 9% after stirring for 11 hours, and the current was 60mA-80mA.
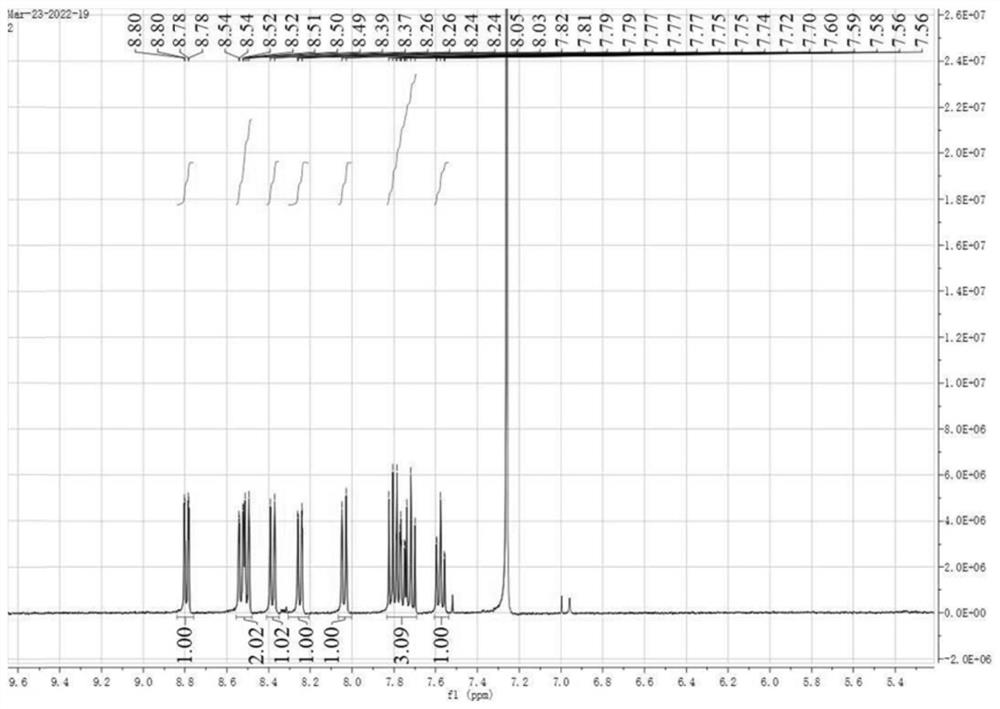
[0044]Transfer the reaction solution to a 250 mL three-neck flask, contin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com