Method for purifying polysaccharides
A technology of polysaccharides and neutral polysaccharides, applied in pharmaceutical formulations, medical preparations containing active ingredients, antibody medical ingredients, etc., can solve the burden of increasing manufacturing compliance, inefficiency, reduced yield and quality consistency, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0099] The following procedure was tested to isolate polysaccharides from Streptococcus cells of two different serotypes (serotypes 3 and 4).
[0100] 1) Precipitate DNA with CTAB and NaCl;
[0101] 2) Depth filtration to remove sediment;
[0102] 3) Precipitation with KI to remove CTAB;
[0103] 4) Centrifuge to remove sediment;
[0104] 5) Carbon filtration to remove protein and other impurities;
[0105] 6) Ceramic hydroxyapatite type (CHT) silica gel column chromatography combined with SARTOBIND phenyl membrane chromatography to further remove impurities;
[0106] 7) Tangential flow filtration for polysaccharide concentration.
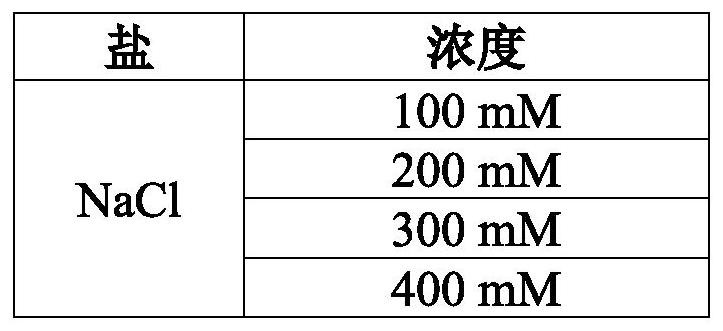
[0107] Reference cells were subjected to a solution containing CTAB and no NaCl. Test cells were subjected to a solution containing CTAB and 400 mM NaCl.
[0108] The total polysaccharide yields are described in Table 1:
[0109] Table 1.
[0110]
[0111] As shown in Table 1, cells contacted with solutions containing NaCl resulted in a ...
example 2
[0120] The following procedure is used to isolate polysaccharides, eg, positively charged, negatively charged and / or neutral polysaccharides (eg, pullulan), from A. pullulans cells. The cells are A. pullulans var. pullulan, A. pullulans var. pullulan, or both. The cells contain one of A. pullulans strain MC 571, strain MC 573, strain MC 574, strain MC576, strain MC 711, strain MC 737, strain MC 745, strain MC 767, strain CBS 701.76 and strain CBS 105.22. one or more.
[0121] 1) Precipitate DNA with CTAB and salt;
[0122] 2) Depth filtration to remove sediment;
[0123] 3) Precipitation with KI to remove CTAB;
[0124] 4) Centrifuge to remove sediment;
[0125] 5) Carbon filtration to remove protein and other impurities;
[0126] 6) Ceramic hydroxyapatite type (CHT) silica gel column chromatography combined with SARTOBIND phenyl membrane chromatography to further remove impurities;
[0127] 7) Tangential flow filtration for polysaccharide concentration.
[0128] The co...
example 3
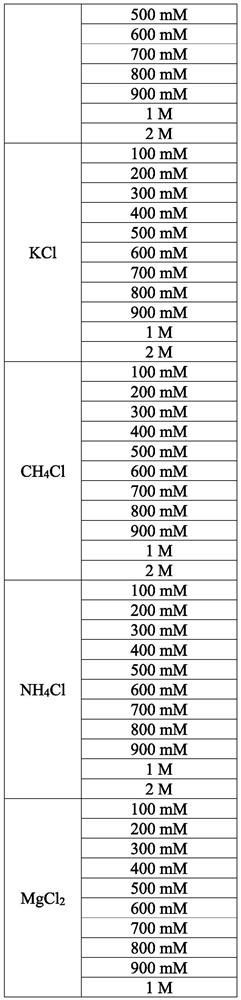
[0130]The procedure described in Example 2 was used to isolate polysaccharides, eg, positively charged, negatively charged and / or neutral polysaccharides, from S. aureus cells. The cells contain one or more of S. aureus serotypes 1, 2, 5, 8, 336 or NT. The concentrations of CTAB used in the procedure are provided in Table 2. The concentrations and types of salts used in the procedure are provided in Table 3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com