Synthesis method of 2, 3-diamino indole compound
A technology of a diaminoindole and a synthesis method, applied in 2 fields, can solve the problems of high reaction temperature, expensive transition metals, unfavorable for industrialized large-scale synthesis, etc., and achieves simple operation, elimination of metal residues, and high economical applicability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
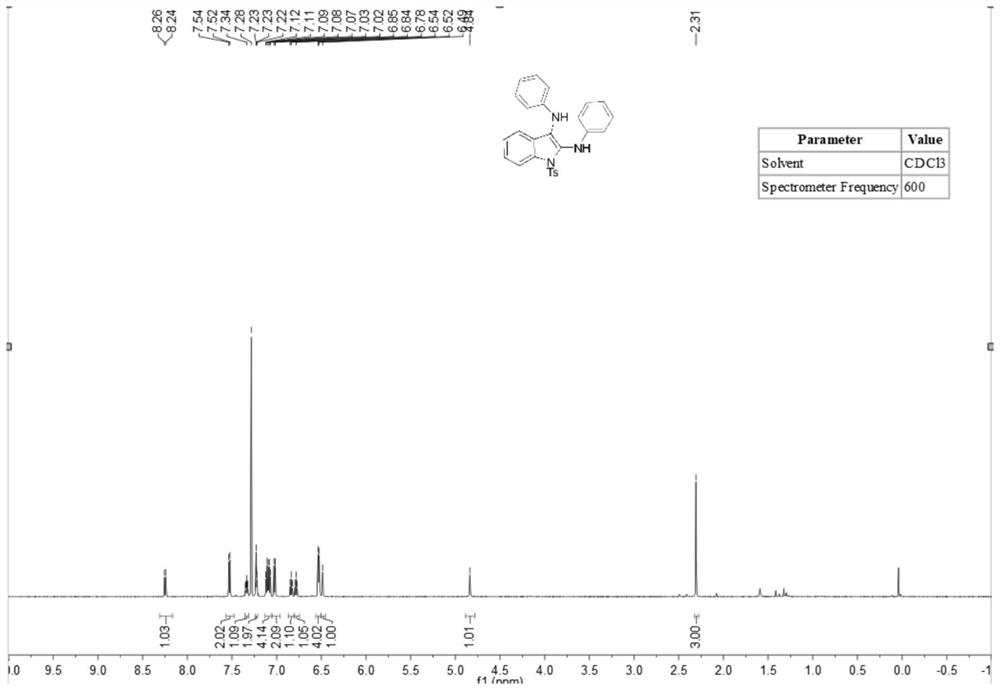
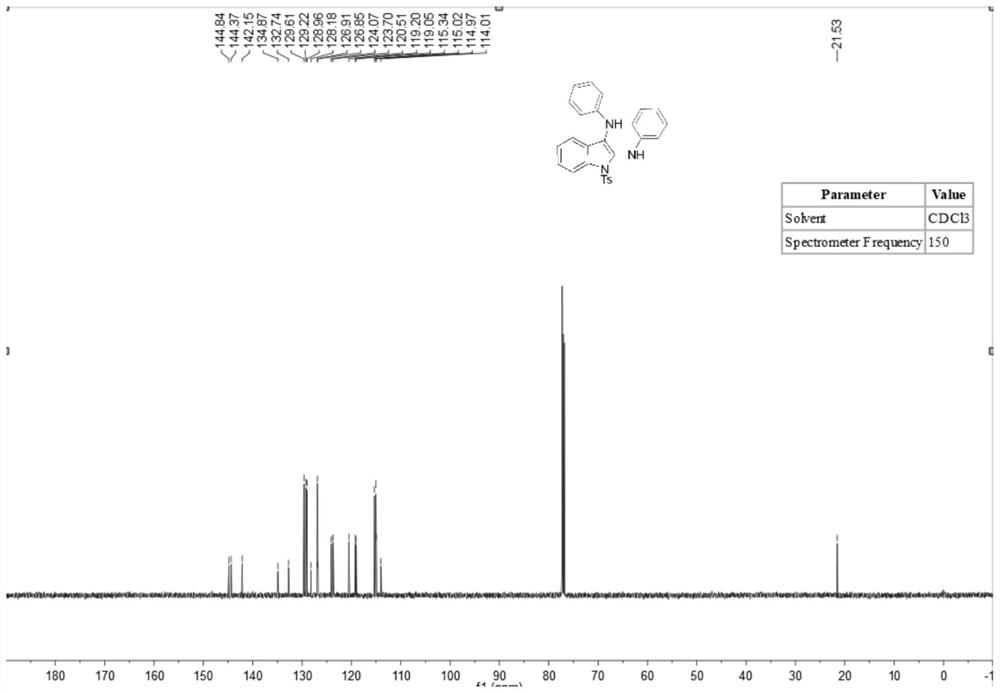
[0036] A method for synthesizing 2,3-diaminoindole compounds comprises the following steps: putting a stirrer into a dry 10mL three-necked flask, adding N-(2-ethynylphenyl) successively under room temperature conditions -4-toluenesulfonamide (83 mg, 0.3 mmol), isopropyl 4-aminobenzoate (219.4 mg, 1.2 mmol), tetrabutylammonium iodide (23 mg, 0.06 mmol), lithium perchlorate (83 mg, 0.75 mmol), tetramethylpiperidine oxide (2.2 mg, 5 mol%), potassium carbonate (2 equiv.), water (1.0 ml) and acetonitrile (5.0 ml). At room temperature, the platinum electrode was used as the anode and the carbon electrode was used as the cathode, and the reaction was carried out under the condition of 5 mA constant current for 10 hours. After the reaction was completed, the reaction solution was extracted with ethyl acetate, and the extracted reaction solution was transferred to a 50 ml eggplant shape In the bottle, use a Heidolph rotary evaporator, the rotation speed is 80-100rpm, the temperature is...
Embodiment 2
[0044] A stirring bar was placed in a dry 10mL there-necked flask, and N-(2-ethynylphenyl)-4-toluenesulfonamide (83mg, 0.3mmol), isopropyl 4-aminobenzoate were added successively at room temperature. (219.4 mg, 1.2 mmol), tetrabutylammonium iodide (23 mg, 0.06 mmol), lithium perchlorate (83 mg, 0.75 mmol), tetramethylpiperidine oxide (2.2 mg, 5 mol%), potassium carbonate ( 2 equiv), water (1.0 ml) and acetonitrile (5.0 ml). At room temperature, the platinum electrode was used as the anode and the carbon electrode was used as the cathode, and the reaction was carried out under the condition of 5 mA constant current for 10 hours. After the reaction was completed, the reaction solution was extracted with ethyl acetate, and the extracted reaction solution was transferred to a 50 ml eggplant shape. In the bottle, use a Heidolph rotary evaporator, the rotation speed is 80-100rpm, the temperature is 38°C, the vacuum degree is 0.1Mpa, and the process is 3min. The residue is then subje...
Embodiment 3
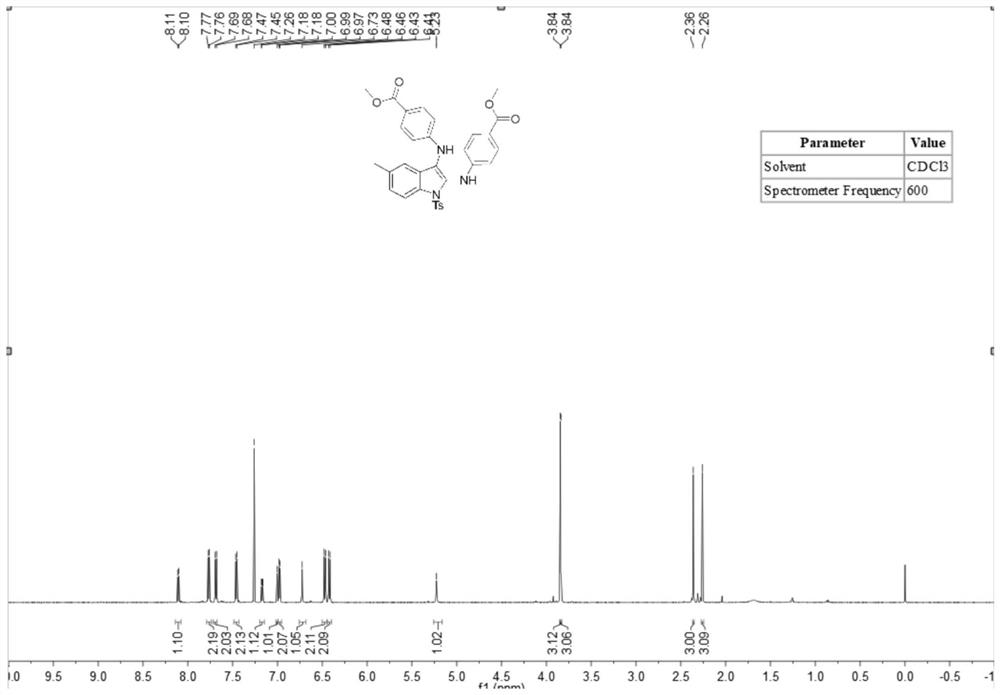
[0052] A stirring bar was placed in a dry 10mL there-necked flask, and N-(2-ethynylphenyl)-4-toluenesulfonamide (83mg, 0.3mmol), methyl 4-aminobenzoate ( 185.3 mg, 1.2 mmol), tetrabutylammonium iodide (23 mg, 0.06 mmol), lithium perchlorate (83 mg, 0.75 mmol), tetramethylpiperidine oxide (2.2 mg, 5 mol%), potassium carbonate (2 equiv), water (1.0 ml) and acetonitrile (5.0 ml). At room temperature, the platinum electrode was used as the anode and the carbon electrode was used as the cathode, and the reaction was carried out under the condition of 5 mA constant current for 10 hours. After the reaction was completed, the reaction solution was extracted with ethyl acetate, and the extracted reaction solution was transferred to a 50 ml eggplant shape In the bottle, use a Heidolph rotary evaporator, the rotation speed is 80-100rpm, the temperature is 38°C, the vacuum degree is 0.1Mpa, and the treatment is 3min. The residue is then subjected to column chromatography using 200-mesh co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com