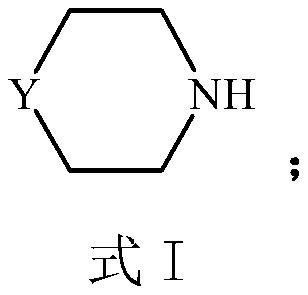
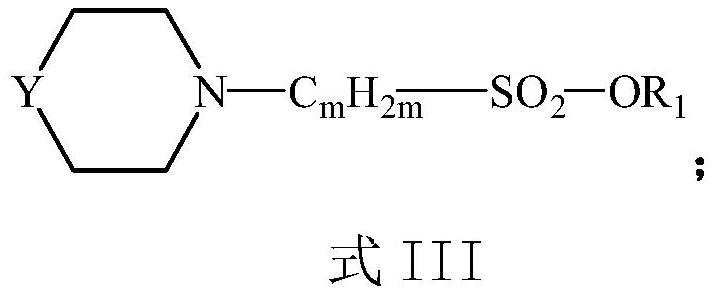
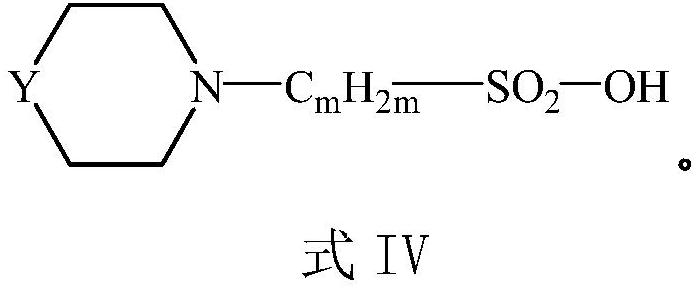
Preparation process of compound containing sulfonic group
A technology containing a sulfonic acid group and a preparation process, applied in the direction of organic chemistry and the like, can solve the problems of large-scale application of compounds hindering the sulfonic acid group, difficulty in obtaining raw materials, and high manufacturing costs, and achieve high product yield, low water content, The effect of increasing productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Preparation of piperazine-N,N'-bis(2-ethanesulfonic acid) disodium (PIPES-2Na)
[0052]
[0053] The preparation of piperazine-N,N'-bis(2-ethanesulfonic acid) disodium comprises the following preparation steps:
[0054] Preparation step S1:
[0055] Under stirring conditions, 100 g of compound 1, compound 2, and reaction solvent acetonitrile were added to a 1L dry reactor, wherein compound 1 was piperazine, and compound 2 was ethyl vinyl sulfonate; the moles of compound 1 and compound 2 were The ratio is 1:4, the reaction temperature is 70°C, the reaction pressure is 0.1MPa (gauge pressure), and the reaction time is 4h.
[0056] After the completion of the reaction, the temperature was lowered to room temperature, the solvent was removed by rotary evaporation of the reaction solution under reduced pressure, and concentrated to obtain a crude product of compound 3.
[0057] Preparation step S2:
[0058] Under stirring conditions, the above 100g compound ...
Embodiment 2
[0063] Example 2: Preparation of piperazine-N,N'-bis(2-ethanesulfonic acid) (PIPES)
[0064]
[0065] The preparation of 3-morpholine propanesulfonic acid comprises the following preparation steps:
[0066] Preparation step S1:
[0067] Under stirring conditions, add 100 g of compound 1, compound 2, and reaction solvent acetone into a 1L dry reactor; wherein, compound 1 is piperazine, and compound 2 is propyl vinylsulfonate; the moles of compound 1 and compound 2 are The ratio was 1:10, the reaction temperature was 40°C, the reaction pressure was 0.2MPa (gauge pressure), and the reaction time was 15h.
[0068] After the completion of the reaction, the temperature was lowered to room temperature, the solvent was removed by rotary evaporation of the reaction solution under reduced pressure, and concentrated to obtain a crude product of compound 3.
[0069] Preparation step S2:
[0070] Under stirring conditions, the above-mentioned 100g of compound 3 was added to a 1L dry ...
Embodiment 3
[0077] Example 3: Preparation of sodium 3-morpholine propanesulfonate (MOPS-Na)
[0078]
[0079] The preparation technology of 3-morpholine propanesulfonate sodium comprises the following preparation steps:
[0080] Preparation step S1:
[0081] Under stirring conditions, add 100 g of compound 1, compound 2, and reaction solvent N,N-dimethylformamide into a 1L dry reactor; wherein, compound 1 is morpholine, and compound 2 is methyl allylsulfonate Ester; the molar ratio of compound 2 and compound 3 is 1:1.5, the reaction temperature is 100° C., the reaction pressure is 0.4 MPa (gauge pressure), and the reaction time is 1 h.
[0082] After the completion of the reaction, the temperature was lowered to room temperature, the solvent was removed by rotary evaporation of the reaction solution under reduced pressure, and concentrated to obtain a crude product of compound 3.
[0083] Preparation step S2:
[0084] Under stirring conditions, the above 100g compound 3 was added to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com