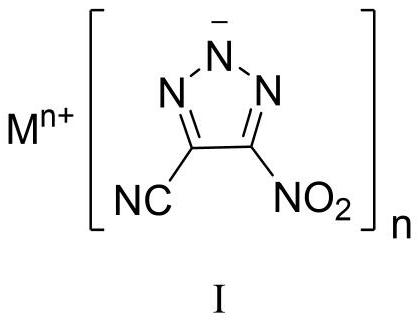
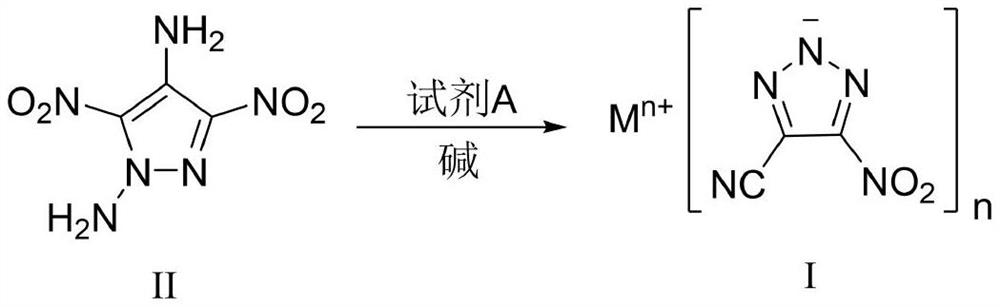
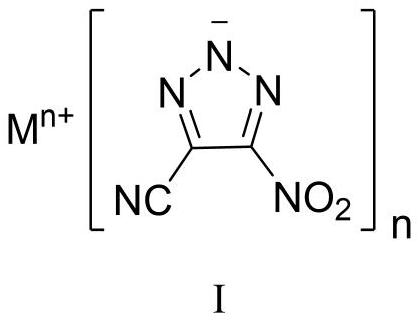
4-cyano-5-nitro-1, 2, 3-triazole metal salt as well as preparation method and application thereof
A technology of metal salt and dinitropyrazole, applied in the field of energetic materials, can solve problems such as poor safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Sodium dichloroisocyanurate (SDIC, 255 mg, 1.16 mmol, 1.5 eq) was dissolved in water (3 mL) at room temperature. Under stirring, 0.35 mL of acetic acid (6.19 mmol, 8.0 eq) was added dropwise thereto, and the mixture was stirred for 0.5 h for use. 1,4-Diamino-3,5-dinitropyrazole (DADNP, 145 mg, 0.77 mmol) was dissolved in 8 mL of acetonitrile, cooled to -5°C, and SDIC / acetic acid aqueous solution was added dropwise while stirring, and the addition was completed in 0.5 h. After the DADNP is consumed, add sodium carbonate to the reaction solution to a pH value greater than 7, and concentrate to obtain a crude product. Column chromatography was performed using silica gel, followed by pure petroleum ether, petroleum ether:ethyl acetate=10:1, then 5:1, 1:1, 1:2 and pure ethyl acetate to obtain pure product, and 94 mg of yellow White powdery solid, yield 57%. 13 CNMR (100MHz, DMSO-d 6 , ppm) δ: 155.73, 114.81, 113.83; IR (cm -1): 3432, 2927, 2856, 2465, 2262, 1545, 1504, 1...
Embodiment 2
[0046] Sodium dichloroisocyanurate (SDIC, 170 mg, 0.77 mmol, 1.0 eq) was dissolved in water (3 mL) at room temperature. Under stirring, 0.35 mL of acetic acid (6.19 mmol, 8.0 eq) was added dropwise thereto, and the mixture was stirred for 0.5 h for use. 1,4-Diamino-3,5-dinitropyrazole (DADNP, 145 mg, 0.77 mmol) was dissolved in 8 mL of acetonitrile, cooled to 0° C., and SDIC / acetic acid aqueous solution was added dropwise while stirring. After the DADNP is consumed, add sodium carbonate to the reaction solution to a pH value greater than 7, and concentrate to obtain a crude product. Use silica gel for column chromatography, followed by pure petroleum ether, petroleum ether: ethyl acetate = 10:1 to 5:1 and 1:1, 1:2 and pure ethyl acetate to obtain pure product, to obtain 86 mg of yellow White powdery solid, yield 52%.
Embodiment 3
[0048] Sodium dichloroisocyanurate (SDIC, 170 mg, 0.77 mmol, 1.0 eq) was dissolved in water (3 mL) at room temperature. Under stirring, 0.35 mL of acetic acid (6.19 mmol, 8.0 eq) was added dropwise thereto, and the mixture was stirred for 0.5 h for use. 1,4-Diamino-3,5-dinitropyrazole (DADNP, 145 mg, 0.77 mmol) was dissolved in 8 mL of acetonitrile, cooled to 5° C., and SDIC / acetic acid aqueous solution was added dropwise while stirring. After the DADNP is consumed, add sodium bicarbonate to the reaction solution to a pH value greater than 7, and concentrate to obtain a crude product. Use silica gel for column chromatography, followed by pure petroleum ether, petroleum ether: ethyl acetate = 10:1, then 5:1 and 1:1, 1:2 and pure ethyl acetate to obtain pure product, and 89 mg of yellow White powdery solid, yield 54%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
| impact sensitivity | aaaaa | aaaaa |
| impact sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com