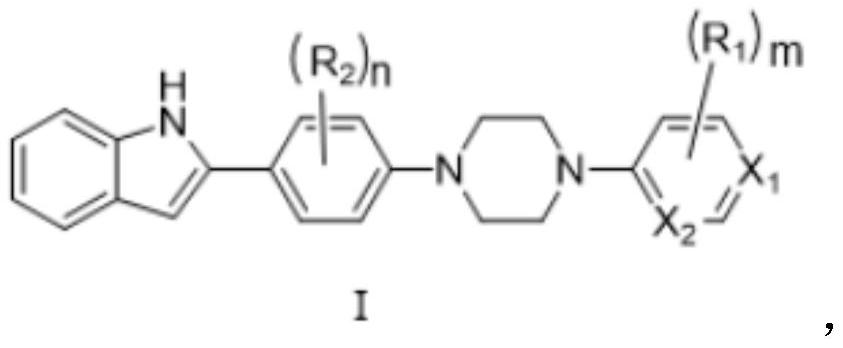
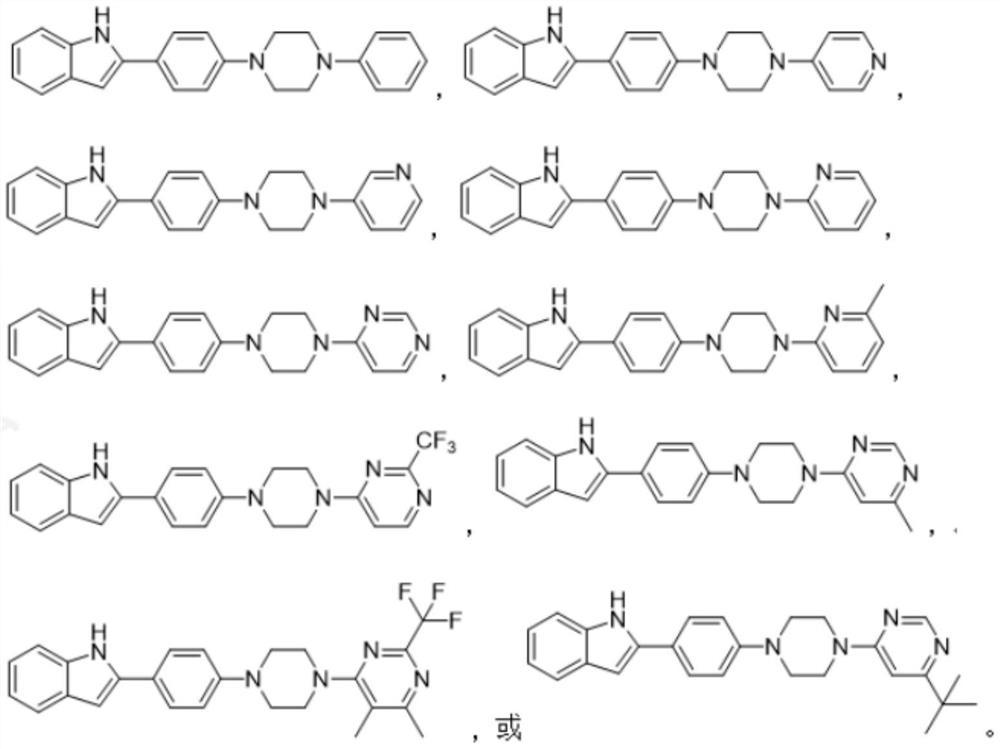
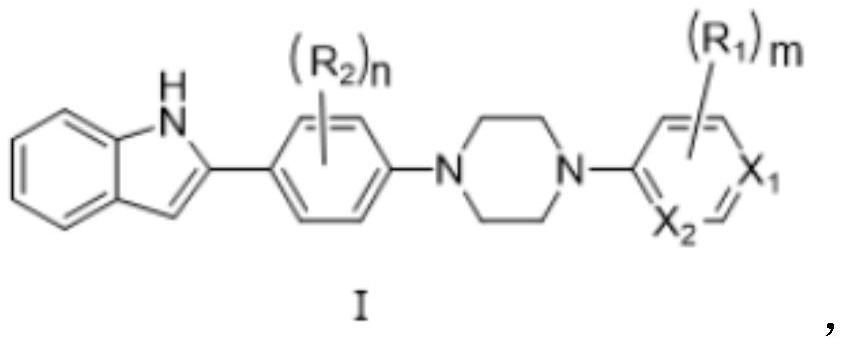
Phenylpiperazine compound and application thereof
A compound and alkyl technology, applied in the field of medicinal chemistry, can solve the problems of peripheral neurotoxicity, side effects, and side effects of chemotherapeutic agents, and achieve good tumor inhibitory activity and high safety effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1 Preparation of 2-(4-(4-phenylpiperazin-1-yl)phenyl)-1H-indole
[0057]
[0058] Step 1 Preparation of Compound 1a
[0059]
[0060] 2-(4-Chlorophenyl)indole (286.8mg, 1.27mmol), piperazine (440.1mg, 4eq), cesium carbonate (620.0mg, 1.5eq) were dissolved in dimethyl sulfoxide (10mL), Stir overnight at room temperature. After monitoring the completion of the reaction, it was diluted with ethyl acetate, washed with saturated brine for 5 times, the organic phase was concentrated, and the compound 1a was isolated by column chromatography. ESI-MSm / z:[M+H] + = 278.1.
[0061] Step 2 Preparation of Compound 1
[0062]
[0063] Compound 1a (278.1 mg, 1 mmol), chlorobenzene (123.8 mg, 1.1 mmol) and cesium carbonate (488.7 mg, mmol) were dissolved in dimethyl sulfoxide (10 mL) and stirred at room temperature overnight. After monitoring the completion of the reaction, it was diluted with ethyl acetate, washed with saturated brine for 5 times, the organic pha...
Embodiment 2
[0064] Preparation of Example 2 Compound 2
[0065]
[0066] The preparation method is the same as the preparation method in Example 1, except that chlorobenzene is replaced by 4-chloropyridine to obtain the title compound. ESI-MS m / z:[M+H] + = 355.2.
Embodiment 3
[0067] Preparation of Example 3 Compound 3
[0068]
[0069] The preparation method is the same as that in Example 1, except that chlorobenzene is replaced by 3-chloropyridine to obtain the title compound. ESI-MS m / z:[M+H] + = 355.2.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap