Methods and products for treating gastrointestinal disorders
A technology for gastrointestinal diseases and diseases, applied in the direction of medical raw materials derived from bacteria, biochemical equipment and methods, and disease diagnosis, which can solve the problems of failing to reduce the relative risk of CDI recurrence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
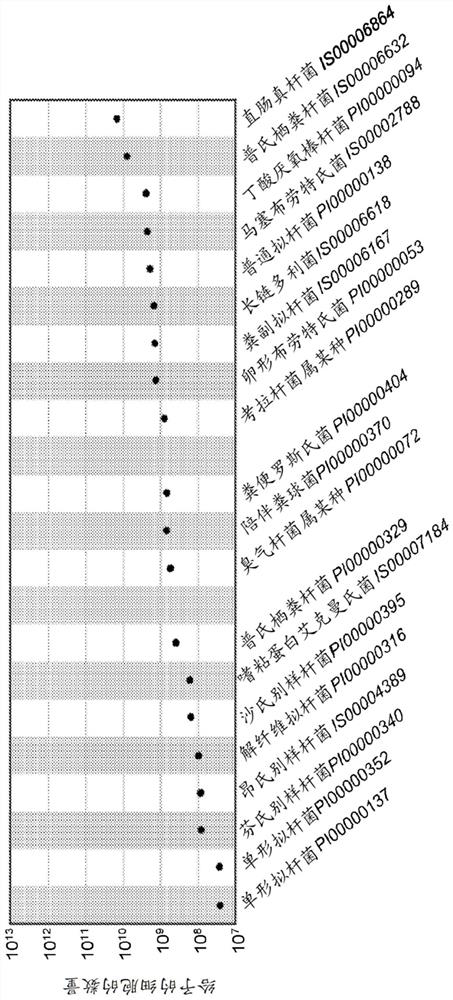
[0640] Embodiment 1: A pharmaceutical composition comprising a mixture of at least two bacterial isolates, wherein at least one of said bacterial isolates comprises at least 95% of the 16S rRNA sequence of the bacterial isolates provided in Table 1 16S rRNA sequence of identity, and wherein said at least two bacterial isolates are isolated from stool samples of different donors.
Embodiment approach 2
[0641] Embodiment 2: The pharmaceutical composition of Embodiment 1, wherein at least two of said bacterial isolates comprise a 16S rRNA having at least 95% identity to the 16S rRNA sequence of the bacterial isolates provided in Table 1 sequence.
Embodiment approach 3
[0642] Embodiment 3: The pharmaceutical composition of Embodiment 1 or Embodiment 2, wherein at least three of said bacterial isolates comprise a 16S rRNA sequence at least 95% identical to the bacterial isolates provided in Table 1 Sexual 16SrRNA sequence.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com