Peptide therapeutics for autoimmune and inflammatory diseases
A technology for autoimmune diseases and inflammatory diseases, applied in allergic diseases, anti-inflammatory agents, non-central analgesics, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Example 1: Materials and Methods
Embodiment 1-1
[0091] Example 1-1: Peptide synthesis, reagents and cell line optimization
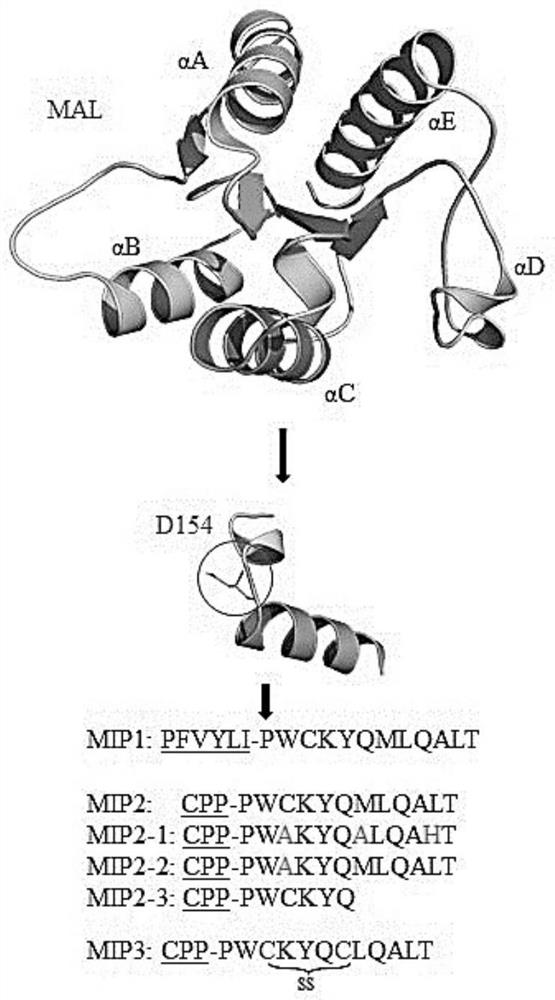
[0092] Peptides were synthesized by Biostem (Ansan, Korea) with 97.09% (MIP1), 97.17% (MIP2) and 95.1% (MIP3) purities, respectively, as determined by reversed-phase high performance liquid chromatography (HPLC; Shimadzu Prominence). All peptides were detected at 220 nm using a Shiseido Capcell pak C18 column (4.6 mm x 50 mm) with a gradient of 10%-60% acetonitrile in 0.1% trifluoroacetic acid (TFA)-water at a flow rate of 1 mL / min. The molecular weights of MIP1, MIP2 and MIP3 were measured by Shimadzu LCMS-2020 and were 2182.6 Da, 3710.5 Da and 3680.5 Da, respectively. LPS (E. coli 0111:B4) and adenosine triphosphate (ATP) were purchased from Sigma-Aldrich. PAM 3 CSK 4 (TLR1 / 2), Poly(I:C)(TLR3), Imiquimod (IMQ; R837, TLR7), R848 (TLR7 / 8) and CpG-ODN (TLR9) were purchased from Thermo Fisher Scientific, Inc., and FSL-1 (TLR2 / 6) were purchased from InvivoGen (San Diego, CA, USA).
[0093] Put HEK-B...
Embodiment 1-2
[0094] Example 1-2: Cell Viability Assay
[0095] Put HEK-Blue TM hTLR4 cells at 5 × 10 4 Density distribution of cells / well, RAW264.7 cells and THP-1 cells were distributed at 2 × 10 5 Density assignment of cells / well. All cells were grown overnight in 96-well plates (BD Biosciences, San Jose, CA, USA). Cell viability was determined using the colorimetric 1-(4,5-dimethylthiazol-2-yl)-3,5-diphenylmethanezan (MTT) assay (Sigma-Aldrich) and / or MTS (3-(4 , 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazole) (Promega Madison, WI, USA) to assay, performed as previously described (Kwon, H.K. et al., Toxicological sciences: an official journal of the Society of Toxicology 148, 204-219 (2015)).
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com