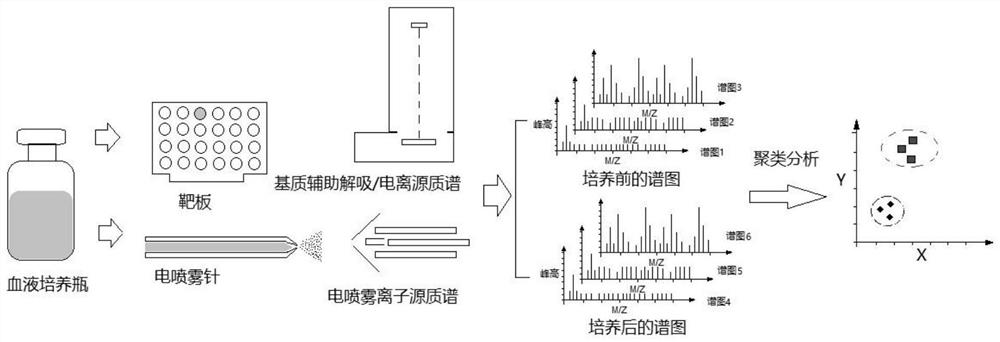
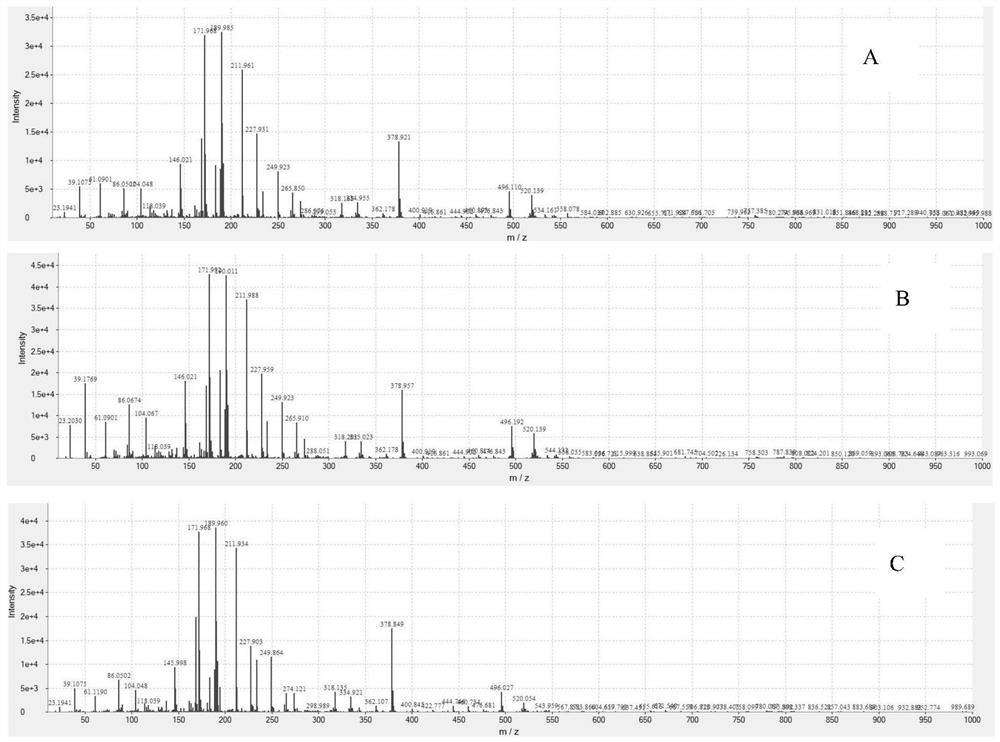
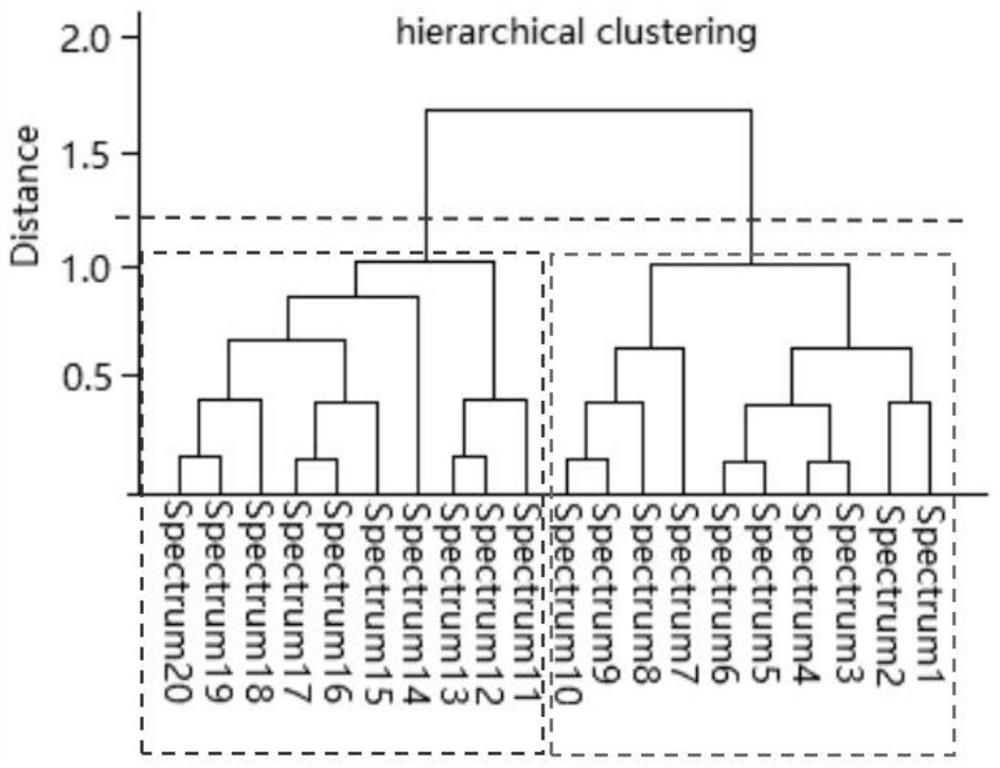
Detection method for blood culture positive reporting
A detection method and blood culture technology, which are applied to measurement devices, preparation of test samples, instruments, etc., can solve the problems of reduced detection sensitivity, high cost of blood culture bottles, high background, shortened reporting time, and simple method. Quick and easy to implement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] In the present invention, the matrix liquid preferably includes the following raw materials: matrix, organic solvent, deionized ultrapure water and trifluoroacetic acid. The matrix preferably includes an organic matrix or an inorganic matrix. The organic matrix preferably includes cinnamic acid or 2,5-dihydroxybenzoic acid. The present invention is not particularly limited to the specific type of the inorganic matrix, and the conventional inorganic matrix types in the art are used. can be. The present invention has no special limitation on the specific source of each raw material in the matrix liquid, and conventional commercially available products in this field can be used. In the present invention, the volume ratio of the organic solvent to deionized ultrapure water is preferably 10:0-1:9, more preferably 7:3, the organic solvent preferably includes acetonitrile, and the deionized ultrapure water The specification of the water is preferably resistivity>18MΩ.cm, and ...
Embodiment 1
[0029] Take the sample (containing liquid medium and blood) in the 500uL blood culture bottle, pour it into the vacuum separation gel blood collection tube, after the blood is completely coagulated, centrifuge at 3000 rpm for 10 minutes to obtain the serum. Extract 100uL of the above serum, use a 3kDa ultrafiltration membrane to remove blood cells and large proteins, and take the supernatant for sample injection.
[0030]Weigh 10 mg of cinnamic acid and dissolve in 100 mL of a mixture of acetonitrile and deionized ultrapure water (V:V=70:30), deionized ultrapure water (resistivity>18MΩ.cm), and finally add 0.11% trifluoro Acetic acid to prepare matrix solution. Mix 50uL of the above supernatant with 50uL of matrix solution to obtain the sample to be tested and wait for sample application.
[0031] Use a pipette to spot 1uL of the above-mentioned sample to be tested on the target plate, 10 spots for each sample. After the drying and crystallization is completed, it is sent to...
Embodiment 2
[0033] Take the sample (containing liquid medium and blood) in the 1000uL blood culture bottle, inject it into the vacuum separation gel blood collection tube, after the blood is completely coagulated, centrifuge at 3000 rpm for 10 minutes to obtain the serum. Take 100uL of the above serum, add methanol organic solvent according to the volume ratio of 1:10, centrifuge at 12000 rpm for 10 minutes, take the supernatant and wait for sample injection.
[0034] Weigh 10mg of 2,5-dihydroxybenzoic acid and dissolve in 100mL of a mixture of acetonitrile and deionized ultrapure water (V:V=1:9), deionized ultrapure water (resistivity>18MΩ.cm), and finally Add 5% trifluoroacetic acid to prepare matrix solution. Mix 50uL of the above supernatant with 50uL of matrix solution to obtain the sample to be tested and wait for sample application.
[0035] The electrospray ion source is used, 1uL is injected each time, and the mass range is set to 10-1000Da. The positive ion mode is also used to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| electrical resistivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap