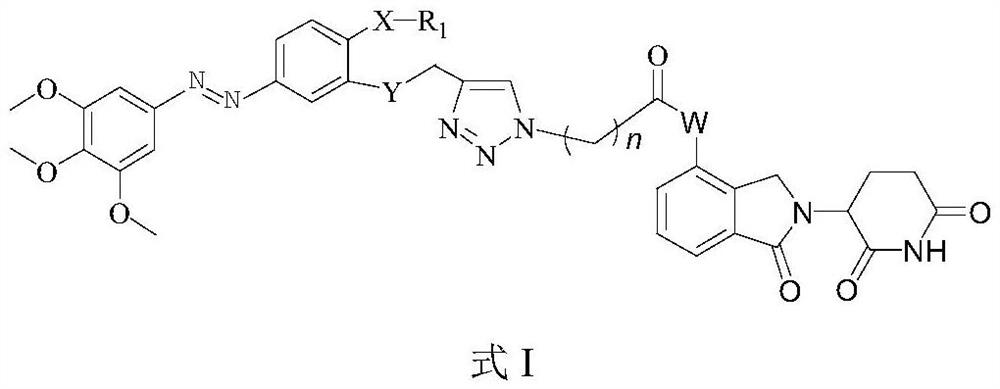
Azo compound for degrading tubulin as well as synthesis method and application of azo compound
A technology of tubulin and compounds, applied in the application field of medicine, can solve the problems such as the defects of acquired drug resistance of tubulin inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The synthesis of embodiment 1 Azo-H
[0038]
[0039] Select a 100ml three-necked flask, add a magnetic rotor, put a thermometer and a constant pressure dropping funnel on it, and place it on a magnetic stirrer. Weigh 1.83g (10mmol) of 3,4,5-trimethoxyaniline, add 5ml of 95% ethanol solution, cool to -10°C with -20°C glycerol, turn on the magnetic stirrer, stir and dissolve, the reaction solution is Bright yellow.
[0040] Measure 2ml of 37% concentrated hydrochloric acid and add it dropwise to the above reaction solution. During the dropwise addition, replace the cooling liquid regularly to ensure that the temperature of the reaction solution is lower than 0°C, and the reaction solution turns into a beige turbid liquid.
[0041] Weigh 1.39g (20mmol) of sodium nitrite, prepare 5ml of aqueous solution, and also place it in cooling liquid to cool. After the hydrochloric acid was added dropwise, the sodium nitrite solution was added dropwise, and the temperature was a...
Embodiment 2
[0045] Synthesis of Example 2 Azo-Me
[0046]
[0047] 0.55 g of Azo-H prepared in Example 1, 0.3 g of potassium carbonate and excess methyl iodide were added to 2 mL of DMF, and the reaction was stirred at room temperature 25° C. overnight. After the reaction was completed, water was added and extracted with ethyl acetate to obtain a brown-yellow solid. 1 H-NMR (CDCl 3 ,ppm):7.66(1H,dd,Ph),7.63(1H,d,Ph),7.23(2H,s,Ph),7.02(1H,d,CH),4.87(2H,f,CH2),3.97 (9H,s,3×OCH3),3.93(3H,s,OCH3).
Embodiment 3
[0048] The synthesis of embodiment 3 Azo-Pr
[0049]
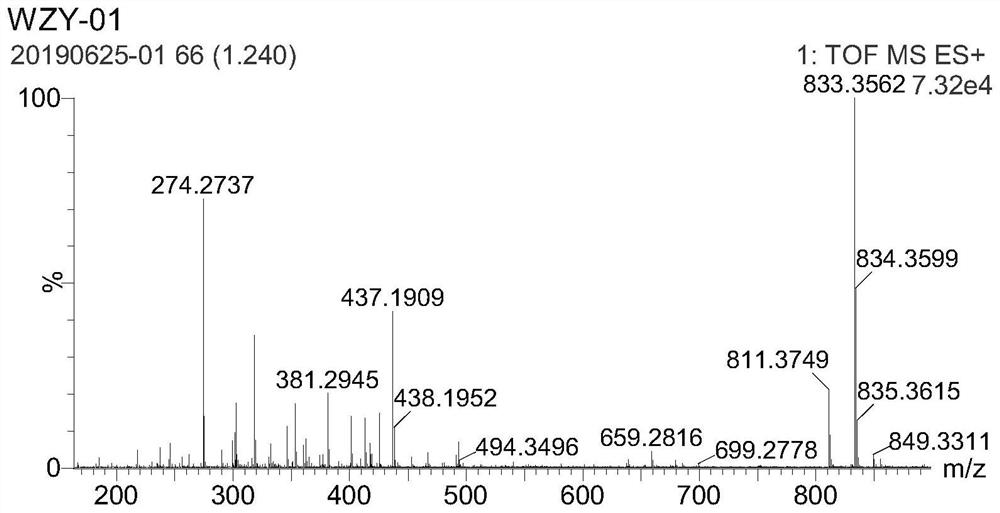
[0050] According to the method described in Example 2, bromopropane was used instead of methyl iodide to obtain Azo-Pr. MS(ESI)m / z:[M+H] + =384.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com