Method for synthesizing thiodicarb with high methomyl conversion rate
A technology of high methomyl conversion rate and synthesis method, applied in the field of thiodicarb synthesis with high methomyl conversion rate, to achieve the effects of increasing reaction temperature, reducing side reactions and improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
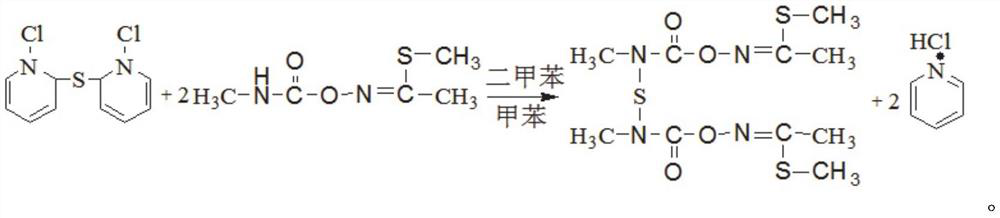
[0055] The invention provides a method for synthesizing thiodicarb with a high conversion rate of methomyl, comprising the steps of:
[0056] Step 1. Preparation of materials: Add xylene, toluene, and pyridine to the synthesis kettle, start stirring, and feed frozen brine into the jacket of the synthesis kettle at the same time; add SCl 2 The liquid is added to the high level tank after metering;
[0057] Step 2. Reaction: observe the temperature change of the synthesis kettle, and start adding SCl dropwise when the temperature in the synthesis kettle drops to -4-0°C 2 , the dropping time is controlled at 40-60 minutes, SCl 2 Control the reaction temperature not higher than 10°C when adding dropwise; SCl 2 After the dropwise addition, continue to stir for 15-30 minutes, add methomyl to the synthesis kettle at one time, feed nitrogen into the kettle until the pressure in the kettle reaches 0.3-0.35MPa, start to heat up, and start timing when the temperature of the kettle reac...
Embodiment 1
[0067] 1. Measure 1010Kg of xylene, 45Kg of toluene and 300Kg of pyridine into the synthesis kettle, start stirring, and at the same time feed frozen brine into the jacket of the synthesis kettle; use a ribbon stirrer in the synthesis kettle; 2 Add 155Kg to the high level tank after weighing;
[0068] 2. When the temperature in the kettle drops to -4°C, start adding SCl dropwise 2 , adding time 45 minutes, SCl 2 During the dropwise addition, the reaction temperature was controlled at 9°C. SCl 2 After the dropwise addition, continue to stir for 25 minutes and then add 450Kg of methomyl to the synthesis kettle at one time, feed nitrogen into the kettle until the pressure in the kettle reaches 0.3MPa, start to heat up, and start timing since the temperature of the kettle reaches 35°C. Reaction at high temperature for 2.5 hours;
[0069] After the reaction is over, use a pump to send the materials in the synthesis tank to the centrifuge. The centrifuge is measured after analy...
Embodiment 2
[0076] 1. Measure 1010Kg of xylene, 45Kg of toluene and 310Kg of pyridine into the synthesis kettle, start stirring, and at the same time feed frozen brine into the jacket of the synthesis kettle; use a ribbon stirrer in the synthesis kettle; 2 Add 160Kg to the high tank after weighing;
[0077] 2. When the temperature in the kettle drops to -3°C, start adding SCl dropwise 2 , adding time 45 minutes, SCl 2 During the dropwise addition, the reaction temperature was controlled at 8°C. SCl 2 After the dropwise addition, continue to stir for 30 minutes and then add 450Kg of methomyl to the synthesis kettle at one time, feed nitrogen into the kettle until the pressure in the kettle reaches 0.32MPa, start to heat up, and start timing since the temperature of the kettle reaches 37°C, at 32°C Reaction at high temperature for 3 hours;
[0078] After the reaction is over, use a pump to send the materials in the synthesis tank to the centrifuge. The centrifuge is measured after analyz...
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com