Method for purifying organic solvents
An organic solvent and hydrophilic technology, which is used in the field of removing metal ion pollutants and non-metal ion pollutants, and can solve the problems of loss of purity of organic solvents and inapplicability of organic solvents.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0048] Some embodiments of the invention are described in detail in the following examples. Then, the following examples are provided to illustrate the present invention in further detail, but should not be construed as limiting the scope of the claims. All parts and percentages are by weight unless otherwise indicated.
[0049] Various terms and nomenclature used in Inventive Examples (Inv.Ex.) and Comparative Examples (Comp.Ex.) are explained as follows:
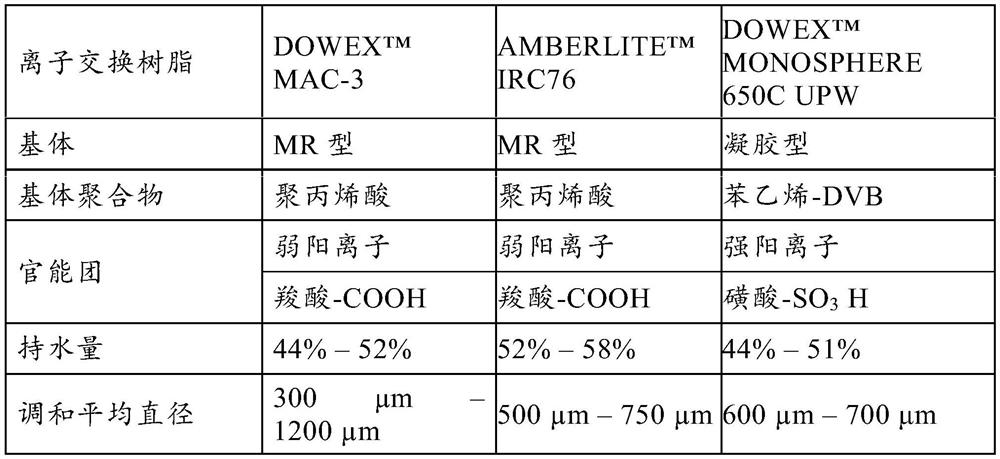
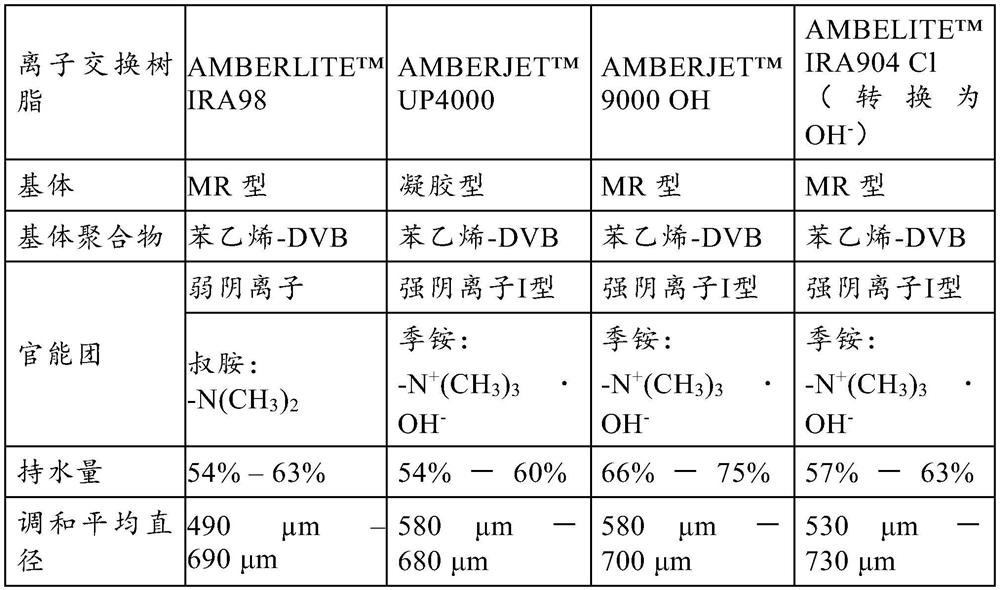
[0050] "DVB" stands for divinylbenzene.
[0051] "MR" stands for Grand Network.
[0052] "BV / hour" stands for bed volume / hour.
[0053] "WAC" stands for Weak Acid Cation Ion Exchange Resin, which has the same meaning as Weak Cation Exchange Resin
[0054] "WBA" stands for Weak Base Anion Ion Exchange Resin and has the same meaning as Weak Anion Exchange Resin.
[0055] "SAC" stands for Strong Acid Cation Ion Exchange Resin and has the same meaning as Strong Cation Exchange Resin.
[0056] "SBA" stands for Strong Base...
example 2
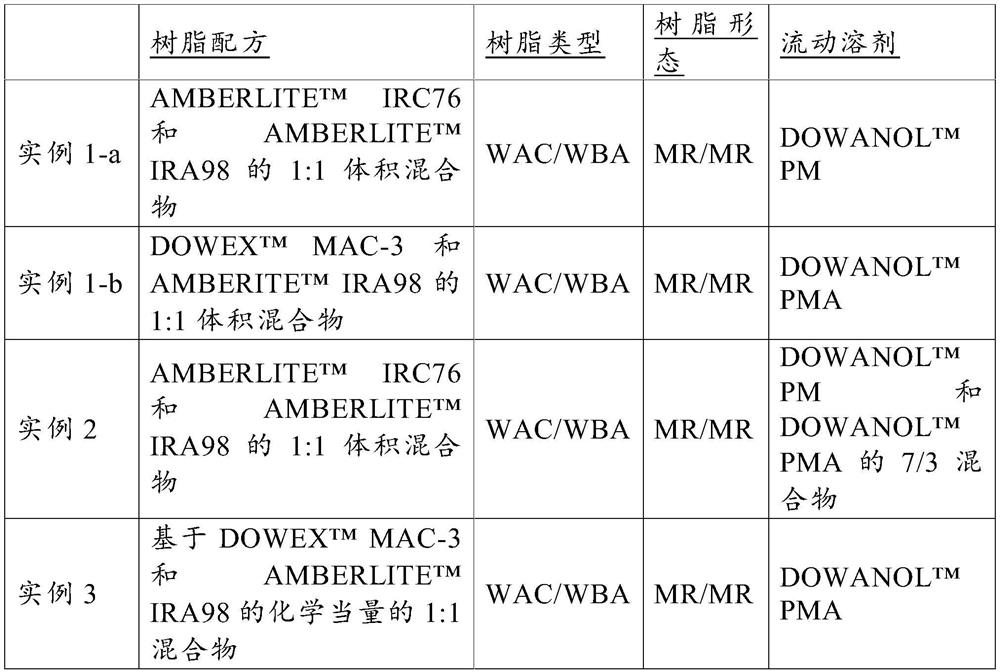
[0072] Example 2 - AMBERLITE with Example 1 for solvent mixture TM IRC76 and AMBERLITE TM IRA98 1:1 volume ratio mixed resin bed
[0073] In this Example 2, 50 mL of the hydrated state of AMBERLITE TM IRC76 resin and 50mL AMBERLITE TM IRA98 resin blend. After flowing ultrapure water at 4 BV / hour for 12 hours, the mixed resin was dried in a vacuum oven (50° C., 10 mmHg, and 24 hours). The dried mixed resin was loaded into a Teflon column with an inner diameter of 50 mm and a length of 150 mm. DOWANOL TM PM and DOWANOL TM A total of 3 L of solvent mixture of PMA was passed through the column at 2 BV / hr for water displacement. Then, start sampling at a flow rate of 4BV / hour, changing the flow rate.
example 3
[0074] Example 3 – for DOWANOL TM DOWEX with Example 1 of PMA TM MAC-3 and AMBERLITE TM IRA98's stoichiometric mixed resin bed
[0075] In this Example 3, 31 mL of hydrated wet DOWEX TM MAC-3 and 89mL Hydration Wet AMBERLITE TM IRA98 mixed. The mixed resin was placed in a vacuum oven at 60° C. and 20 mmHg for a period of 15 hours to prepare a dry resin. The dried resin was packed into a Teflon column. Will DOWANOL TM PMA solvent was flowed through the column at a rate of 8 mL / min for 8 hours. Then, start sampling at a flow rate of 4BV / hour, changing the flow rate.
[0076] Table IV - Comparative Examples
[0077]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com