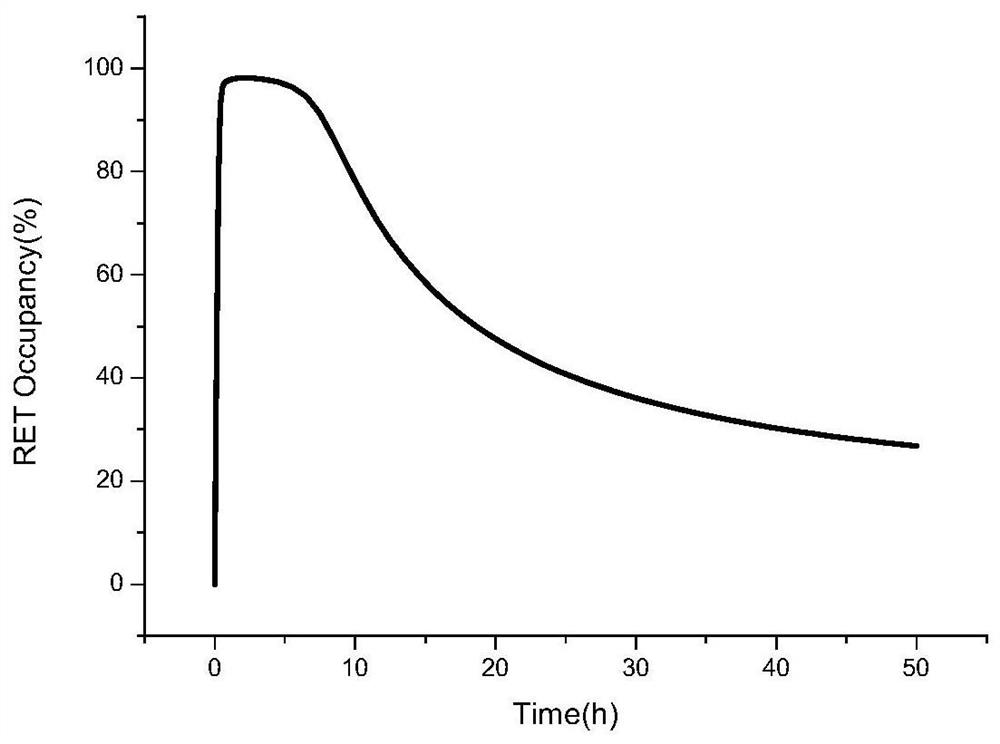
Method for predicting in-vivo PK/PD change after Selpatinib combined medication through PBPK-RO model
A serpa, model technology, applied in the establishment of serpatinib PBPK-RO model, the application of serpatinib tablets in the field of PK and PD effects in the human body, can solve problems such as liver effects, and achieve accurate The effect of high degree and avoiding adverse effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
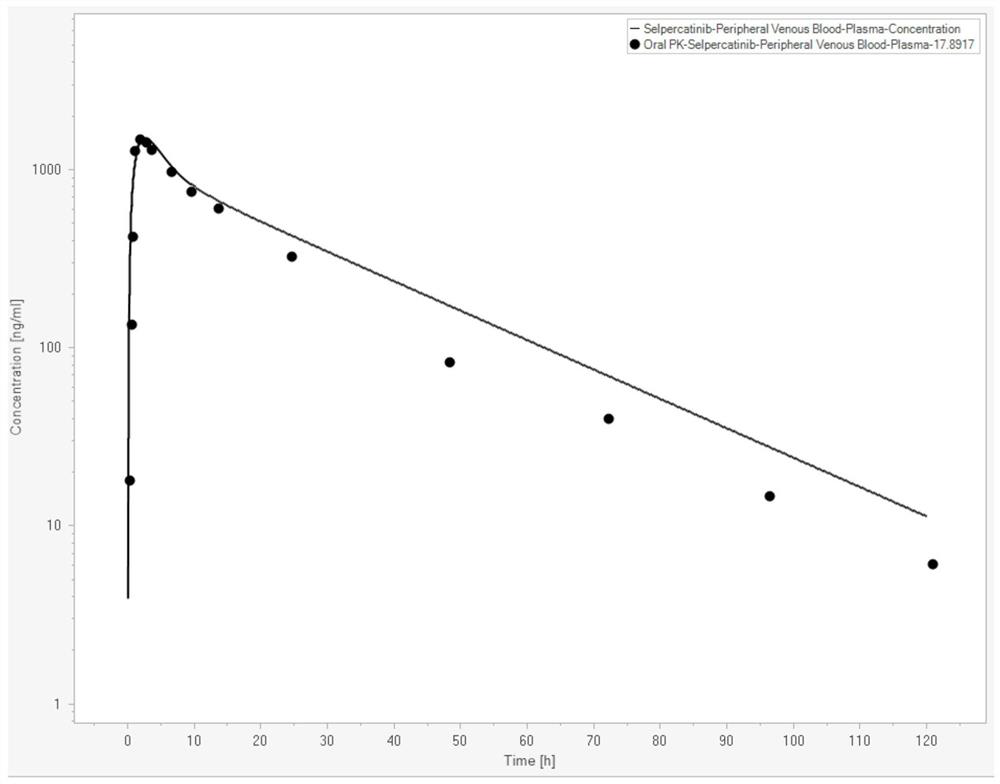
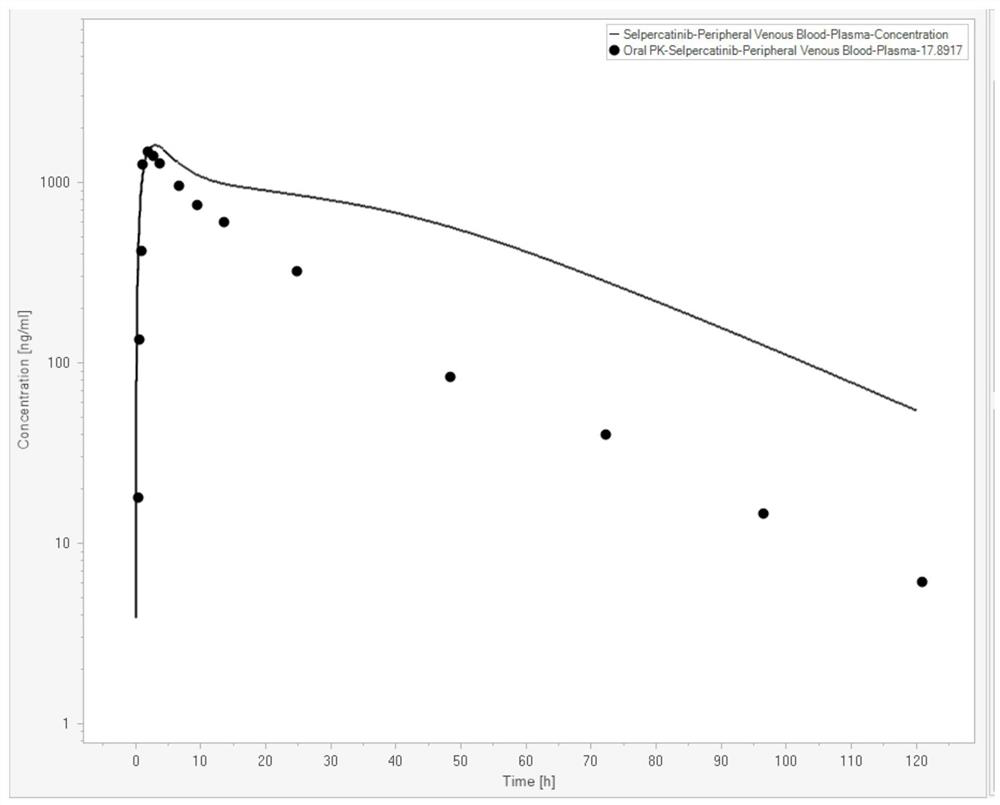
[0074] In this example, the Serpatinib PBPK-RO model was constructed with the help of Berkeley Madonna (Version 10.2.8, Berkeley Madonna, Inc., Albany, CA, USA). Serpatinib basic property parameters are shown in Table 1 (data from FDA review documents,
[0075] https: / / www.accessdata.fda.gov / drugsatfda_docs / nda / 2020 / 213246Orig1s000MultidisciplineR.pdf). The in vivo PK curve data of Serpatinib are shown in Table 2 (data from FDA review documents). The parameters of human physiological attributes used in the PBPK model are shown in Table 3 (the simulated population is American male Caucasian, the average age is 30 years old, the average weight is 80.4Kg, the BMI index is 25.2, and the data comes from the built-in software).
[0076] Table 1
[0077]
[0078]
[0079] Table 2
[0080] time (h) Concentration (ng / mL) 0.25 17.89 0.5 133.96 0.8 419.69 1.0 1267.92 2.0 1482.26 3.0 1410.88 4.0 1285.93 8.0 964.68 10.0 750....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com