Norovirus nucleic acid detection kit and use method thereof
A technology for detecting kits and viral nucleic acids, which is applied in the field of norovirus nucleic acid detection, can solve the problems of easy error cost and slow efficiency, and achieve the effects of improving stability, ease of use, and improving sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
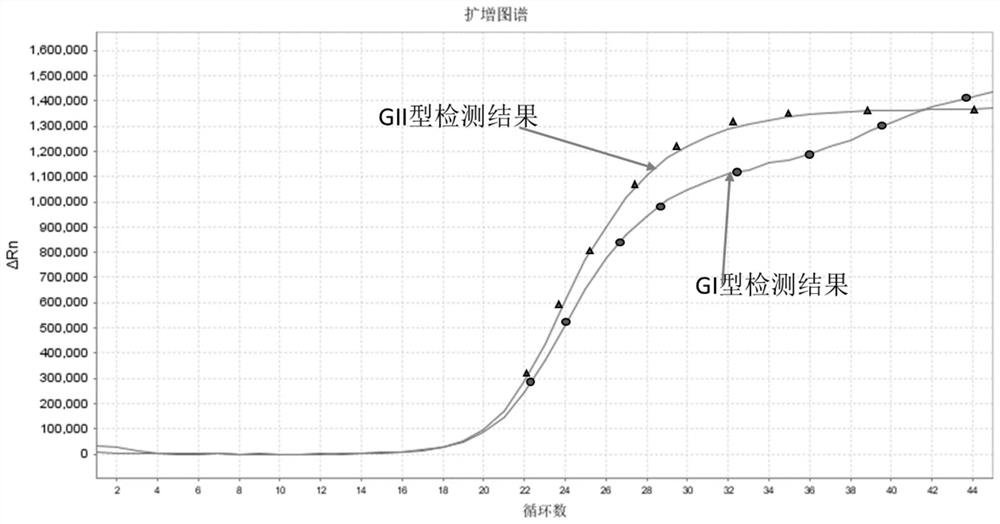
[0113] Norgin virus's classification effect verification: If a Nor is a virus GI (FAM fluorescent group mark, BHQ1 quenching group mark) and GII type (Vic fluorescent group mark, BHQ1 quenching group mark) probe setting Different passages, the kit can be developed as a non-virus GI and GII type typing test reagents, for 10 samples (inactivated clinical samples from GI infection from Shanghai CDC), No. 10) and No. 11 sample (From the inactivation clinical sample of GII infection from Shanghai CDC, No. 11) is detected, and the GI-type and GII-type amplification curves correspond to different fluorescence channels, indicating that fluorescence of corresponding and label probes. Channel, can effectively distinguish between GI and GII types, as shown figure 1 .
Embodiment 3
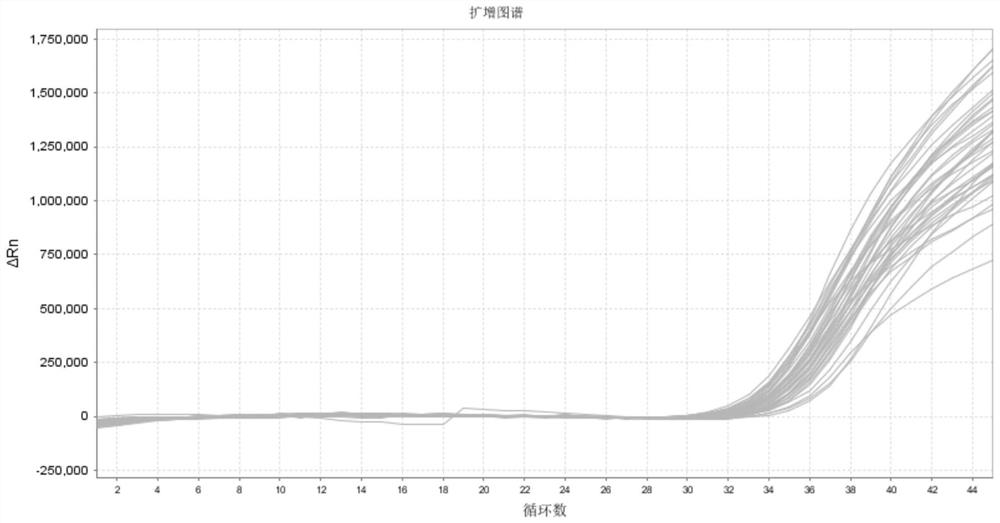
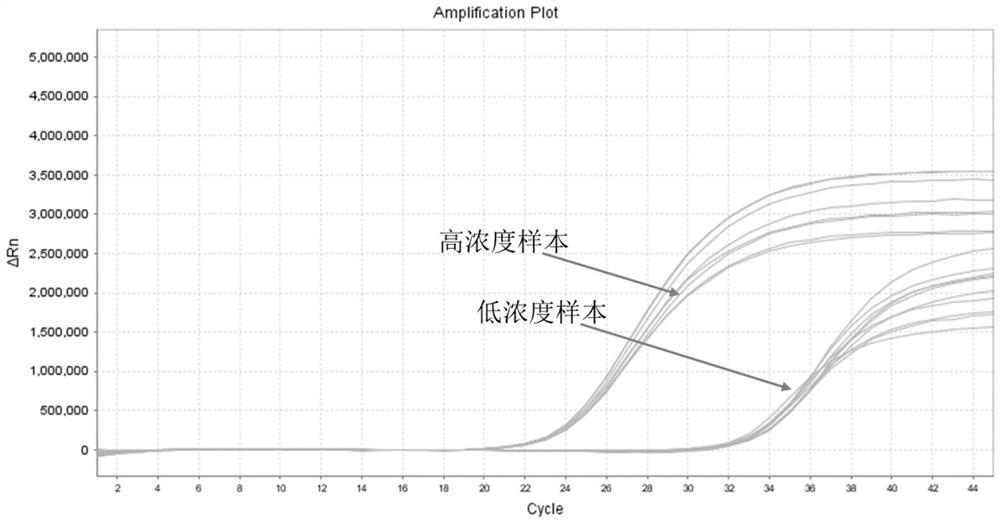
[0115] Detection and precision verification of clinical samples: The Norgin Virus GI is set to the GII probe, and the kit is set to a non-Virus GI and GII-type universal detection reagents, for 74 cases of clinical samples (originating from The GI and / or GII-type inventive clinical samples of the Shanghai CDC, numbered 1-74, Table 2), and diluted to 50 copies / ml (detection limit concentration) to be detected. The detection results of Table 2 can be seen that the kit of the present invention can effectively detect the non-virus GI and GII type samples of the detection limit concentration, and the detection rate is 100%, indicating that it is 50 copies / ml for clinical samples. The results of its detection are shown in Figure 2. Dilute 10-20 samples to 100 copies / ml (low concentration) and 10 5 COPIES / ML (high concentration), its amplification curve has no abnormalities, CT value of CV is 0.8% and 0.6%, and it is less than 5%, indicating that its precision is qualified, and...
Embodiment 4
[0119] Specificity Verification (Cross Reaction): The specific detection effect of other pathogens is detected in Table 3. It is negative that other common pathogens are detected.
[0120]
[0121]
[0122] table 3
[0123] Specific verification (anti-interference): The kit detection control group and the addition of different interference substances were detected, and the comparative test results of the Nori virus Gi type were added, and the comparative test results of the interference substance and the control group (non-interference substance) were shown in Table 4. . The results show that when the Norgin Virus GI sample contains the interference material in the following table, there is no interference to the kit, indicating that the reagent box anti-interference ability is strong.
[0124]
[0125]
[0126] Table 4: Novoli virus GI interference substance research experimental data
[0127] Specific verification (anti-interference): The kit is detected the control grou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com