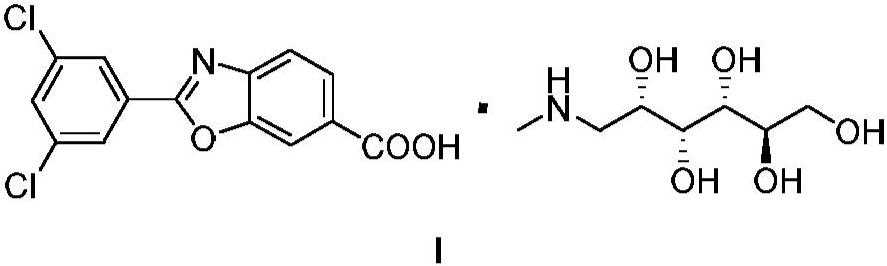
Process for preparing 1-deoxy-1-methylamino-d-glucitol 2-(3,5-dichlorophenyl)-6-benzoxazolecarboxylate
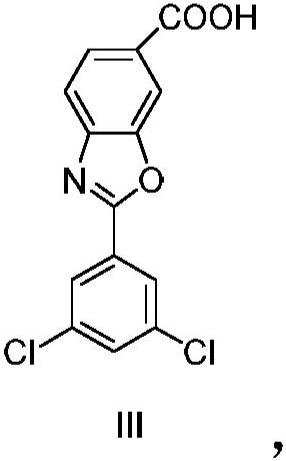
A kind of technology of benzoxazole carboxylate, dichlorophenyl, be applied in the potassium salt of 2-benzoxazole-6-carboxylate, 6-carboxy-2--benzoxazole meglumine or chlorphenazole Meglumine acid, the preparation of key intermediate 4--3-hydroxybenzoic acid methyl ester, the field of the manufacture of meglumine meglumine salt of chlorphenazole acid, can solve the problems of lack of production methods, expensive drugs, increased cost and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1
[0151] Reference Example 1: Preparation of 4-(3,5-dichlorobenzamido)-3-hydroxybenzoic acid methyl ester
[0152] Methyl 4-amino-3-hydroxybenzoate (2.0 g, 12.0 mmol) was dissolved in dry CH at a temperature of 20 °C to 25 °C 2 Cl 2To the suspension in (20 mL) was added pyridine (1.2 mL, 15.0 mmol). The mixture was cooled to a temperature between 0°C and 5°C for 15 minutes. Then, a solution of 3,5-dichlorobenzoyl chloride (2.7 g, 12.6 mmol) was added. The resulting suspension was warmed at a temperature between 20°C and 25°C and the reaction was stirred for 16 hours. Once the reaction was complete, the viscous suspension was filtered and washed with CH 2 Cl 2 washing. Suspend the obtained brown solid in acetone / HO 2 O in a mixture and the slurry was stirred at room temperature for one hour; the solid was filtered, washed with a mixture of acetone and water, and dried in an air oven at 45°C to afford HO as a light brown solid slightly purified by TLC. Methyl 4-(3,5-dichlo...
Embodiment 1
[0154] Example 1: Synthesis of 4-(3,5-dichlorobenzamido)-3-hydroxybenzoic acid methyl ester
[0155] To a suspension of methyl 4-amino-3-hydroxybenzoate (50.0 g, 300 mmol) in dry THF (400 mL) was added NaHCO at a temperature between 20 °C and 25 °C 3 (27.6 g, 330 mmol). The mixture was cooled to a temperature of 10°C to 15°C, and a solution of 3,5-dichlorobenzoyl chloride (65.8 g, 314 mmol) in dry THF (100 mL) was added dropwise, keeping the internal temperature below 20°C. Then, the temperature was adjusted to 20°C to 25°C, and the reaction mixture was stirred for 16 hours. Once the reaction was complete, the solvent was vacuum distilled and the resulting crude was resuspended in a 500 mL mixture of acetone (250 mL) and water (250 mL). The viscous suspension was stirred at room temperature for one hour and at 0°C to 5°C for another hour. The resulting solid was filtered, washed with a mixture of acetone and water, and dried in an air oven at 45°C to afford 4-(3,5-dichlorob...
Embodiment 2
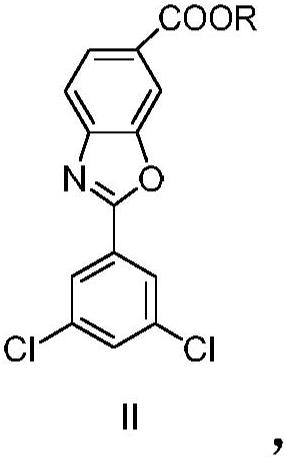
[0157] Example 2: 2-(3,5-dichlorophenyl)benzo[d] Synthesis of oxazole-6-carboxylate methyl ester
[0158] P-toluenesulfonic acid monohydrate (4.5g, 23.52mmol) was added to 4-(3,5-dichlorobenzamido)-3-hydroxybenzoic acid methyl ester (80g, 235.2mmol) obtained in Example 1 ) in suspension in toluene (1.6 L). The resulting mixture was heated at reflux and the reaction was stirred with elimination of water for 16 hours. The solution was cooled to 45°C and filtered. The solvent was distilled under vacuum and the resulting crude was resuspended in acetone (560 mL) and water (80 mL). The resulting viscous suspension was stirred at room temperature for one hour, the solid was filtered, washed with a mixture of acetone and water, and dried in an air oven at 45 °C to afford 2-(3,5-di Chlorophenyl) benzo[d] Azole-6-carboxymethyl ester (70.0 g, 217.0 mmol).
[0159] Yield: 92.4%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com