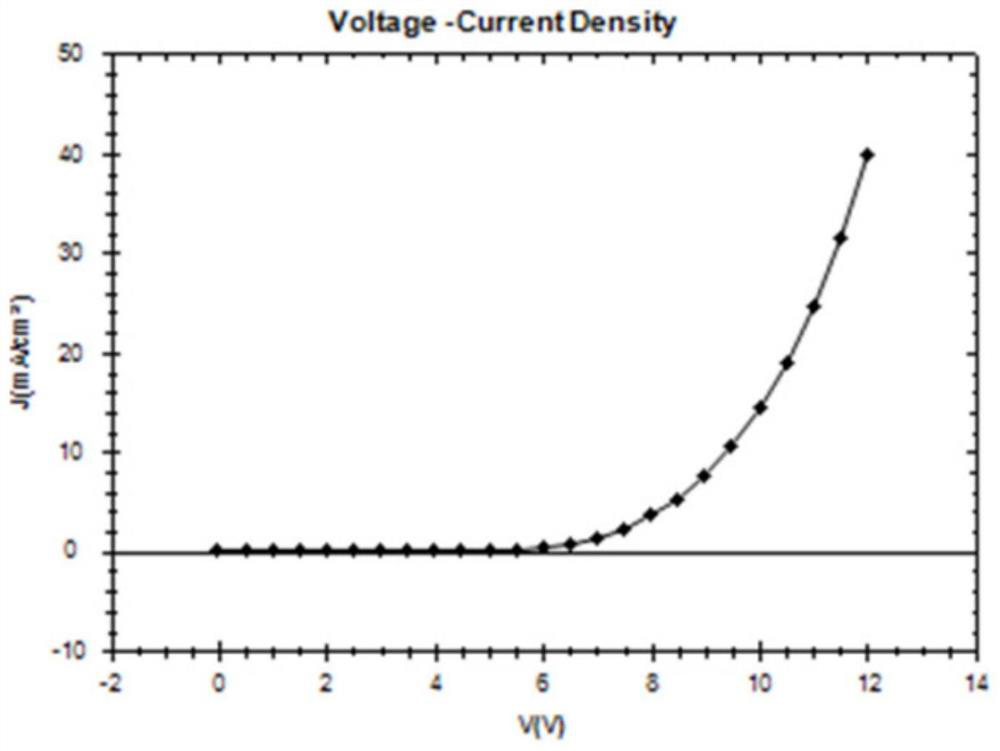
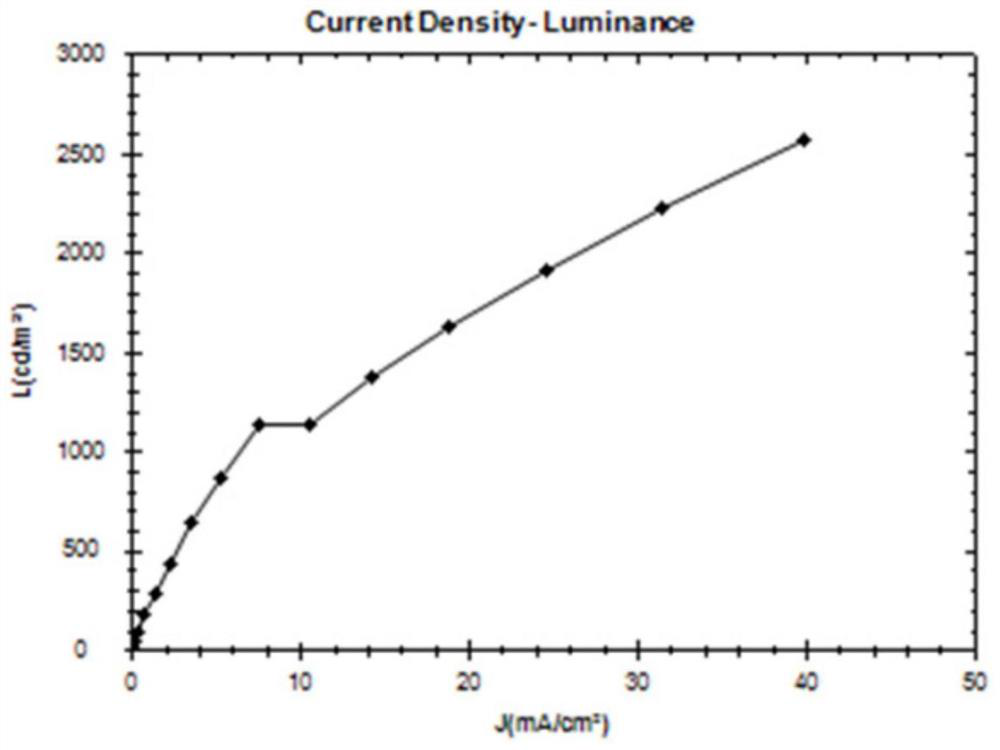
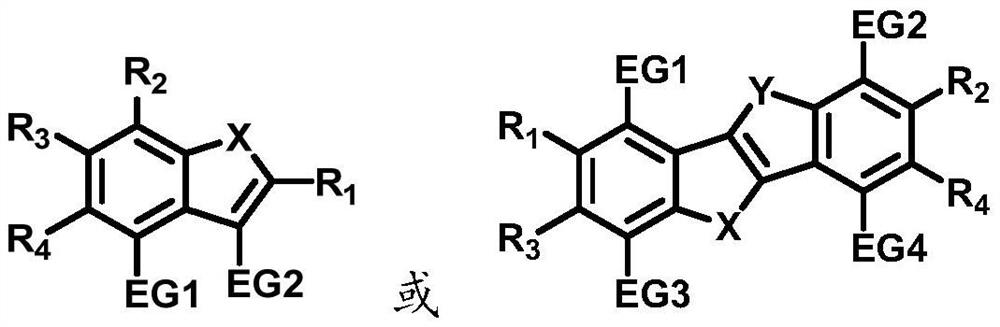
Bipolar fluorescent material based on benzo five-membered heterocycle, preparation method of bipolar fluorescent material and organic electroluminescent device
A technology of fluorescent material and five-membered heterocyclic ring, applied in the field of organic optoelectronics, can solve the problem of low luminous efficiency of devices, and achieve the effects of high luminous efficiency, improved unipolar characteristics, and balanced charge injection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] This example provides a bipolar fluorescent material based on a benzo five-membered heterocycle, which is compound 1, and the synthetic route of compound 1 is as follows:
[0073]
[0074] The synthetic method of compound 1 specifically comprises the following steps:
[0075] Synthesis of Intermediate 1: Take raw material 1 (1.73g, 10mmol), raw material 2 (2.36g, 12mmol), potassium carbonate (4.14g) and toluene (50mL) into a round bottom flask, react at 100°C for 12 hours, Add 50mL phosphoric acid aqueous solution, continue to stir for 2 hours, use chloroform to extract, the aqueous phase is extracted three times with 60mL ethyl acetate, combine organic phase, dry over anhydrous sodium sulfate, crude product is subjected to silica gel column chromatography (petroleum ether / ethyl acetate 6 / 1 (volume ratio)) to obtain Intermediate 1 (0.83 g, 42% yield).
[0076] Synthesis of Intermediate 2: Take a 200mL double-necked round-bottomed flask, connect it with a spherical c...
Embodiment 2
[0080] This example provides a bipolar fluorescent material based on a benzo five-membered heterocycle, which is compound 39. The synthetic route of compound 39 is as follows:
[0081]
[0082] The synthetic method of compound 39 specifically comprises the following steps:
[0083] Synthesis of intermediate 1: as described in compound 1, except that starting material 1 (2.36 g, 10 mmol) was used to obtain intermediate 1 (1.17 g, yield 45%).
[0084] Synthesis of Intermediate 2: Take a 200mL double-necked round-bottom flask, connect a spherical condenser to it, fill it with nitrogen after drying, add Intermediate 1 (3.62 g, 10 mmol), raw material 3 (5.57 g, 12 mmol), tetratriphenyl Palladium phosphine (183 mg, 0.2 mmol), 2 mol / L potassium carbonate aqueous solution (10 mL), toluene (100 mL). Heated to reflux for 24 hours, cooled to room temperature, the solution was poured into water, extracted with chloroform, the aqueous phase was extracted three times with ethyl acetate,...
Embodiment 3
[0088] This example provides a bipolar fluorescent material based on a benzo five-membered heterocycle, which is compound 48, and the synthetic route of compound 48 is as follows:
[0089]
[0090] The synthetic method of compound 48 specifically comprises the following steps:
[0091] Synthesis of intermediate 1: as described in compound 1, except that starting material 1 (1.73 g, 10 mmol) was used to obtain intermediate 1 (0.77 g, yield 39%).
[0092] Synthesis of intermediate 2: as described in compound 1, except that starting material 3 (2.51 g, 12 mmol) was used to obtain intermediate 2 (2.86 g, yield 88%).
[0093] Synthesis of intermediate 3: as described in compound 1, yield 98%.
[0094] Synthesis of Intermediate 4: Take Intermediate 3 (4.04g, 10mmol), raw material 4 (2.15g, 12mmol), potassium carbonate (4.14g) and DMF (50mL) into a round bottom flask, and react at 70°C for 12 hours , cooled to room temperature, the solution was poured into water, extracted with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com