Light and thin anti-shrinkage urinary incontinence sling mesh and preparation method thereof
A technology for urinary incontinence and sling, applied in the field of medical devices, can solve the problems of increased discomfort, low longitudinal elongation, and easily shrinkable and deformed tissues, and achieves the advantages of improving patient comfort, reducing foreign body sensation, and improving surgical safety. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The embodiment of the present application also provides a method for preparing the aforementioned urinary incontinence sling mesh, comprising the following steps:
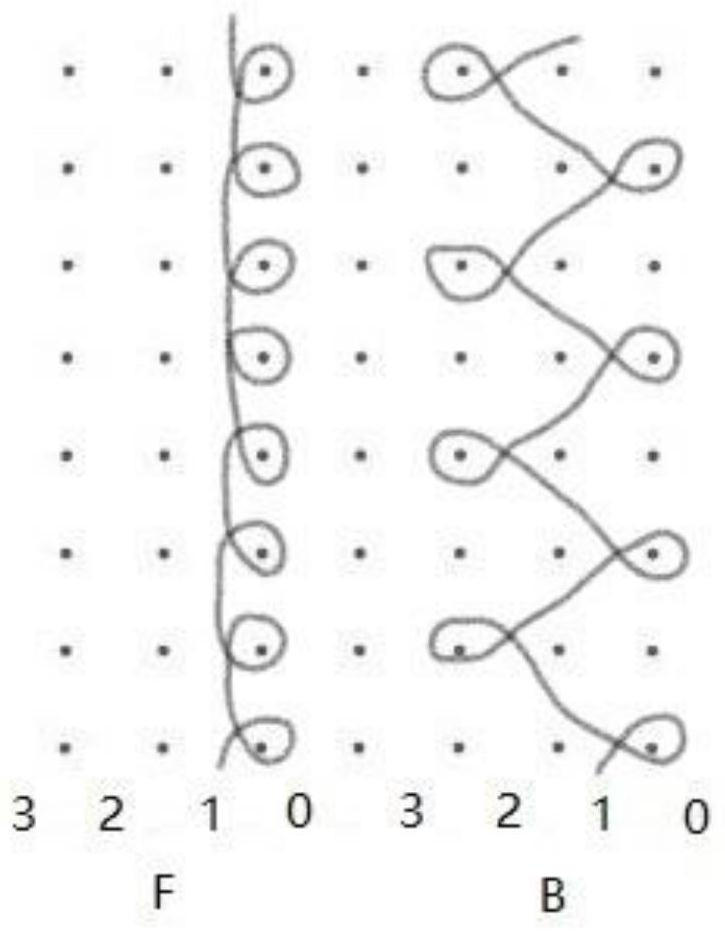
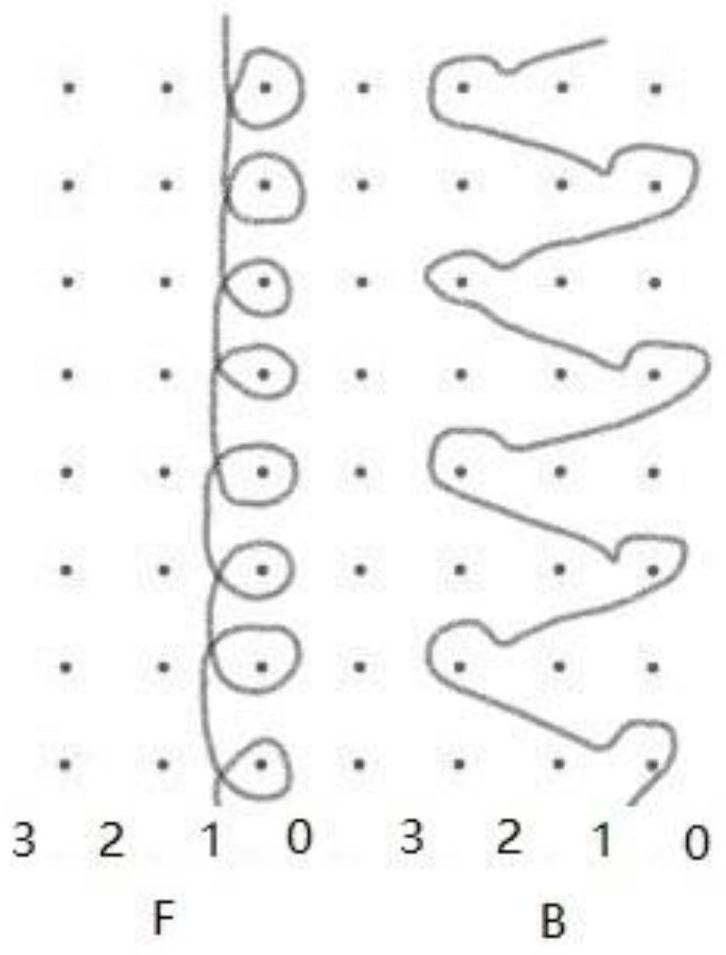
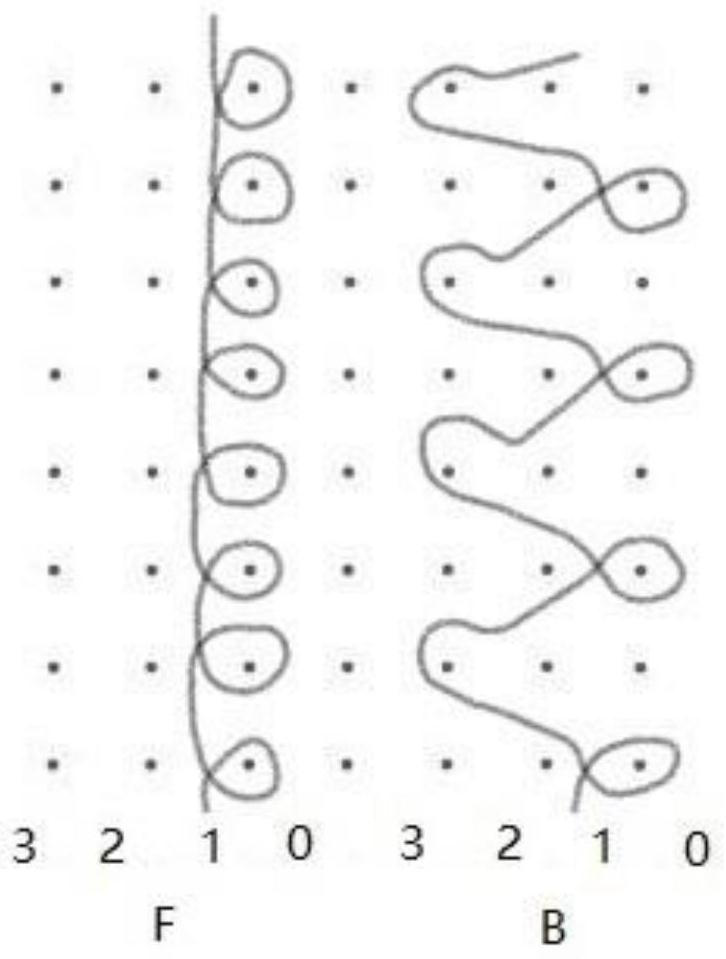
[0027] Two sets of yarn guide combs are installed on the warp knitting machine. Two groups of monofilaments are respectively threaded on the two yarn guide combs, and they are all arranged with one thread and one empty. The filaments are lapped longitudinally on the corresponding knitting needle; each monofilament of the second group is looped longitudinally on the two adjacent knitting needles of the corresponding first group of lapping needles. Laying the yarn in turn to form a loop, weaving to obtain a light and thin anti-shrinkage urinary incontinence sling mesh.
[0028] The urinary incontinence sling mesh provided by this application is light in weight and small in thickness, which can reduce the sensation of foreign body in the body. In addition, the mesh structure is stable and the elongation is low,...
Embodiment 1
[0049] Selection of raw materials for light and thin anti-shrinkage sling mesh:
[0050] In this embodiment, medical polypropylene monofilament is selected as a raw material, and its properties are shown in Table 1.
[0051] Table 1 Material selection and performance
[0052] Raw material type diameter (mm) Breaking strength (gf / d) Elongation at break (%) PP monofilament 0.13 5.2 18
[0053] Weaving process parameters of the light and thin anti-shrinkage sling mesh: the light and thin anti-shrinkage sling mesh is woven by a warp knitting machine, and the specific machine process is shown in Table 2.
[0054] Table 2 weaving process parameters
[0055]
[0056] Performance data of light and thin anti-shrinkage sling mesh:
[0057] The test method of breaking strength and elongation refers to GB / T 3923.1 "Textile Fabric Tensile Properties Part 1: Determination of Breaking Strength and Elongation at Break (Strip Method)", and the test method of t...
Embodiment 2
[0064] Selection of raw materials for light and thin anti-shrinkage sling mesh:
[0065] In this embodiment, medical polyvinylidene fluoride monofilament is selected as the raw material, and its properties are shown in Table 5.
[0066] Table 5 Material selection and performance
[0067] Raw material type diameter (mm) Breaking strength (gf / d) Elongation at break (%) PVDF monofilament 0.15 3 26
[0068] Weaving process parameters of the light and thin anti-shrinkage sling mesh: the light and thin anti-shrinkage sling mesh is woven by a warp knitting machine, and the specific machine process is shown in Table 6.
[0069] Table 6 weaving process parameters
[0070]
[0071] Performance data of light and thin anti-shrinkage sling mesh:
[0072] After post-processing such as sizing etc., carry out performance test (test method is the same as embodiment 1) to the mesh sheet that weaves according to described laying yarn track, test result is as fol...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap