Method for differentiating human induced pluripotent stem cells into natural killer cells
A technology of natural killer cells and pluripotent stem cells, applied in the biological field, can solve problems such as difficulty in expanding scale and complicated operation, and achieve the effect of strong cell killing ability, strong cytokine secretion ability and tumor killing ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0143] The verification of embodiment 1 culture medium I
[0144] Control other parameters unchanged, only adjust the composition of medium I, select the basic medium AIM-V, and adjust cytokine I, as shown in Table 1. The results of flow cytometry are shown in Table 2, and the detection diagram is shown in figure 2 .
[0145] Among them, the components of medium II are AIM-V, SCF 30ng / ml, TPO 10ng / m, FLT3L 10ng / ml, IL1520ng / ml.
[0146] The composition of medium III is PRIME-XV, SCF 10ng / ml, IL7 10ng / ml, Human Serum Albumin 20%, human AB serum 15%.
[0147] Table 1 Verification of medium Ⅰ
[0148]
[0149]
[0150] Table 2 D39 flow cytometry results
[0151]
Embodiment 2
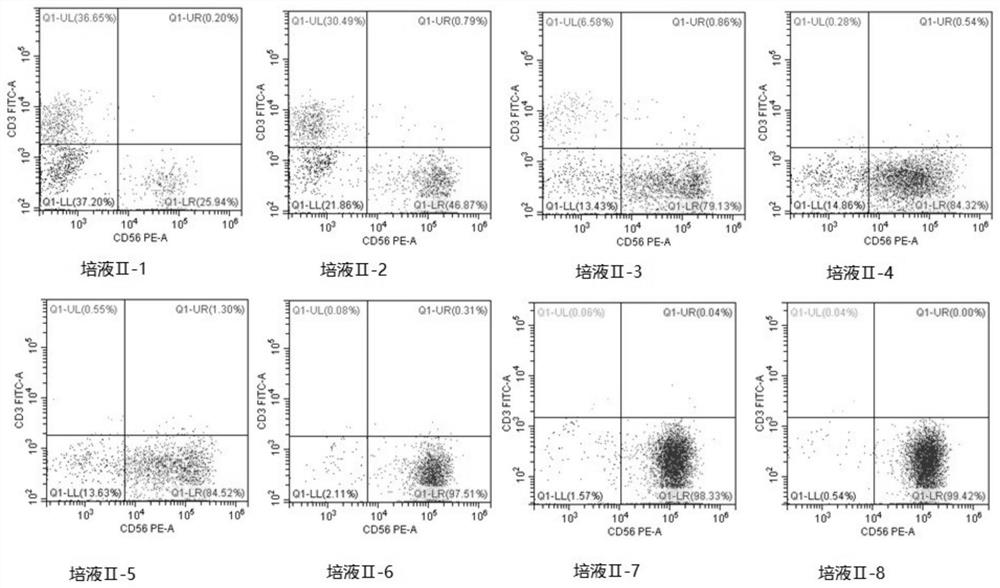
[0152] Verification of embodiment 2 medium II
[0153] Control other parameters unchanged, only adjust the composition of the medium II, select the basic medium AIM-V, adjust the cytokine II, as shown in Table 3, the results of the flow cytometry test are shown in Table 4, and the test figure is shown in image 3 .
[0154] Components of medium I: AIM-V, VEGF 40ng / ml, bFGF 20ng / ml, SCF 50ng / ml, IL-3 10ng / ml.
[0155] The composition of medium III is PRIME-XV, SCF 10ng / ml, IL7 10ng / ml, Human Serum Albumin 20%, human AB serum 15%.
[0156] Table 3 Validation of Medium II
[0157] Element SCF (ng / ml) FLT3L (ng / ml) IL12 (ng / ml) IL18(ng / ml) IL21(ng / ml) Culture medium II-1 \ \ 10 50 15 Culture medium II-2 20 25 \ \ \ Culture medium II-3 0 25 10 50 15 Culture medium II-4 20 25 0 50 15 Culture medium II-5 20 200 10 50 15 Culture medium II-6 1 35 25 1 200 Culture medium II-7 20 25 10 50 15 ...
Embodiment 3
[0161] Verification of Example 3 Medium III
[0162] Control other parameters unchanged, only adjust the composition of the medium III, select the basal medium AIM-V, and adjust the cytokine II, as shown in Table 5. The results of flow cytometry are shown in Table 6, and the detection diagram is shown in Figure 4 .
[0163] Components of medium I: AIM-V, VEGF 40ng / ml, bFGF 20ng / ml, SCF 50ng / ml, IL-3 10ng / ml.
[0164] Components of medium II: Optimizer, SCF 30ng / ml, FLT3L 30ng / ml, IL12 10ng / ml, IL1810ng / ml, IL21 10ng / ml.
[0165] Table 5 Verification of Medium III
[0166]
[0167] Table 6 flow detection results
[0168]
[0169] Embodiment 4 killing detection
[0170] Controlling other conditional parameters unchanged, only adjusting the components of medium I, medium II and medium III, and detecting NKG2D receptors by flow cytometry.
[0171] Verify that the medium composition is as follows:
[0172] Control group 1:
[0173] Medium I: PRIME-XV, SCF 20ng / ml;
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com