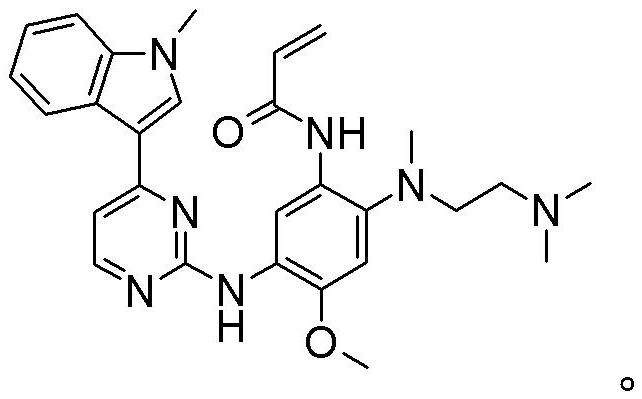
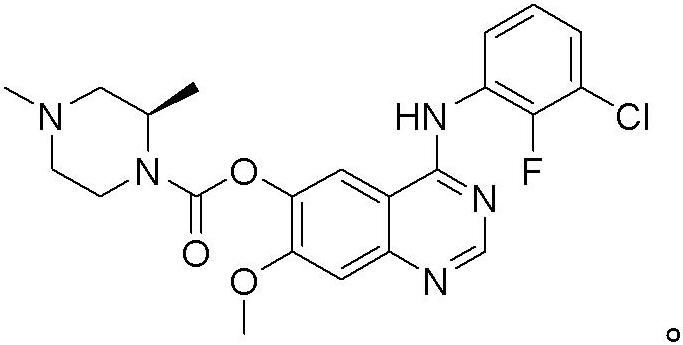
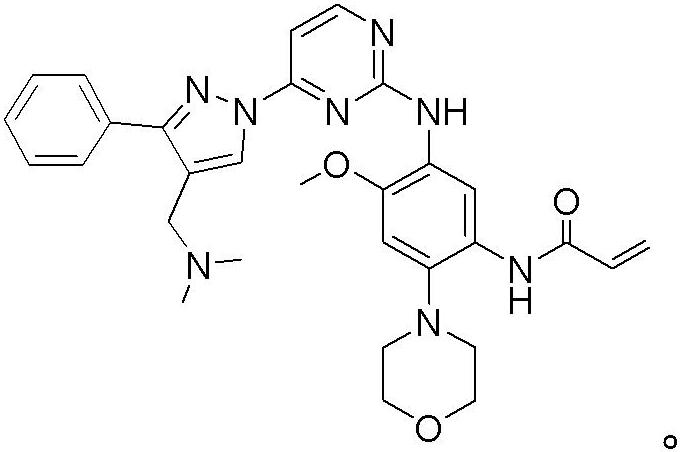
Osimertinib for use in treatment of non-small cell lung cancer
A use and chemotherapy technology, applied in the field of osimertinib for the treatment of non-small cell lung cancer, which can solve the problems that cannot be directly applied, unmet medical needs, and EGFR mutation-positive NSCLC cannot be cured.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0098] A phase III, randomized, double-blind, placebo-controlled, multicenter, international study of the combination of osimertinib and chemotherapy as first-line treatment in patients with locally advanced or metastatic EGFR mutation-positive NSCLC.
[0099] The study title is, "A Phase III, Open-label, Randomized Study of Osimertinib with or without Platinum Plus Pemetrexed Chemotherapy, as First-line Treatment in Patients with Epidermal Growth Factor Receptor (EGFR) Mutation-Positive, Locally Advanced or Metastatic Non-small Cell Lung Cancer [a phase III trial of osimertinib with or without platinum plus pemetrexed chemotherapy as first-line treatment in patients with epidermal growth factor receptor (EGFR) mutation-positive, locally advanced or metastatic non-small cell lung cancer , open-label, randomized study]".
[0100] A follow-up study was conducted to confirm the benefit of the combination of osimertinib, pemetrexed, and platinum chemotherapy in the treatment of pa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com