Process for the preparation of degarelix
A technology of degarelix and linkamide, applied in the field of peptide synthesis, can solve problems such as rearrangement of dihydroorotic acid limiting deprotection mixture
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0231] The following examples provide detailed experimental parameters suitable for the preparation of degarelix of the present invention, and these examples are intended to illustrate but not limit all possible embodiments of the present invention.
[0232] All materials, solvents and reagents were obtained from best grade commercial suppliers and used without further purification unless otherwise stated.
[0233] The solid-phase synthesis of peptides is carried out using common peptide synthesizers, such as Biotage Syrowave instrument (automatic synthesis) and Biotage MultiSynTech (semi-automatic synthesis).
[0234] HPLC analysis was performed on an Agilent Technologies 1200 or 1290 Infinity II instrument using columns C8 Zorbax Eclipse Plus (4.6 x 50 mm, 1.8 μm) or Waters Aquity UPLC BEH C18 (150 mm x 3 mm; 1.7 μm, respectively). The molar yield (%) was calculated considering the final number of moles obtained (based on the assay) divided by the initial number of moles. T...
example 1
[0235] Example 1 : General procedure for degarelix stability experiments in the presence of organic bases: DBU, pyrrolidine, piperidine, TBA, Screening of N-methylpiperazine and Morpholine
[0236] Purified degarelix with <0.15% hydantoin-degarelix impurity (II) content was dissolved in a mixture of DMF and the selected amine at room temperature to obtain a peptide concentration of 130 mg / ml. After 20 minutes, 1 hour 40 minutes and 20 hours, an aliquot of the solution was analyzed by HPLC.
[0237] At the same time, the stability of degarelix was tested after adding 5% water to each sample.
[0238] Results are reported in Table 1 of the specification as HPLC peak area % for hydantoin-degarelix impurity (II).
example 2
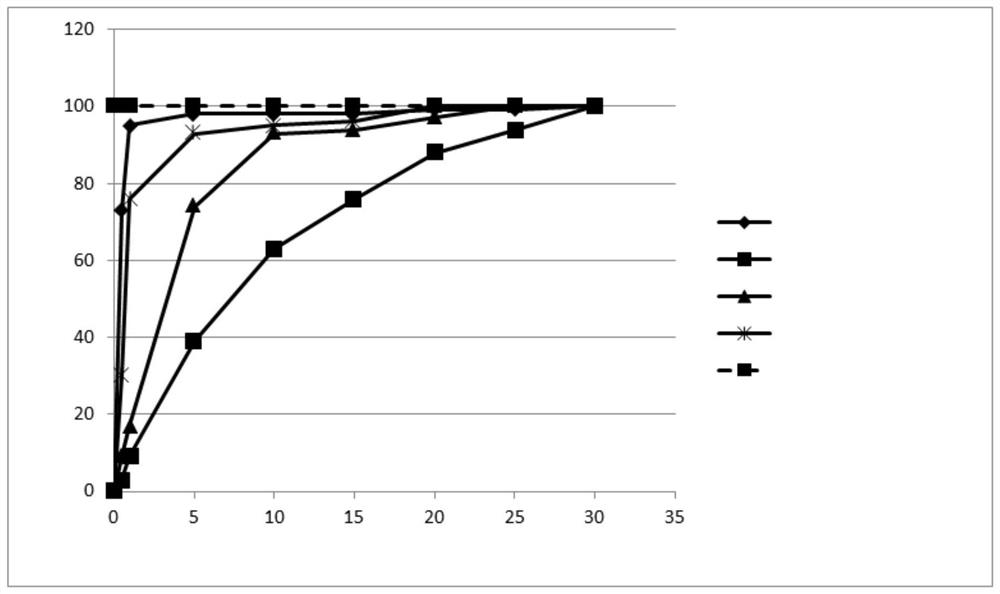
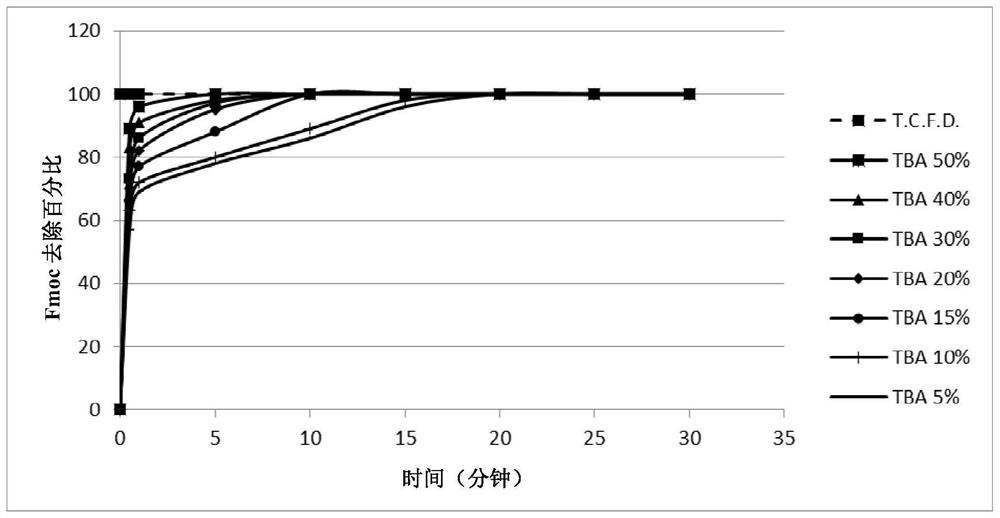
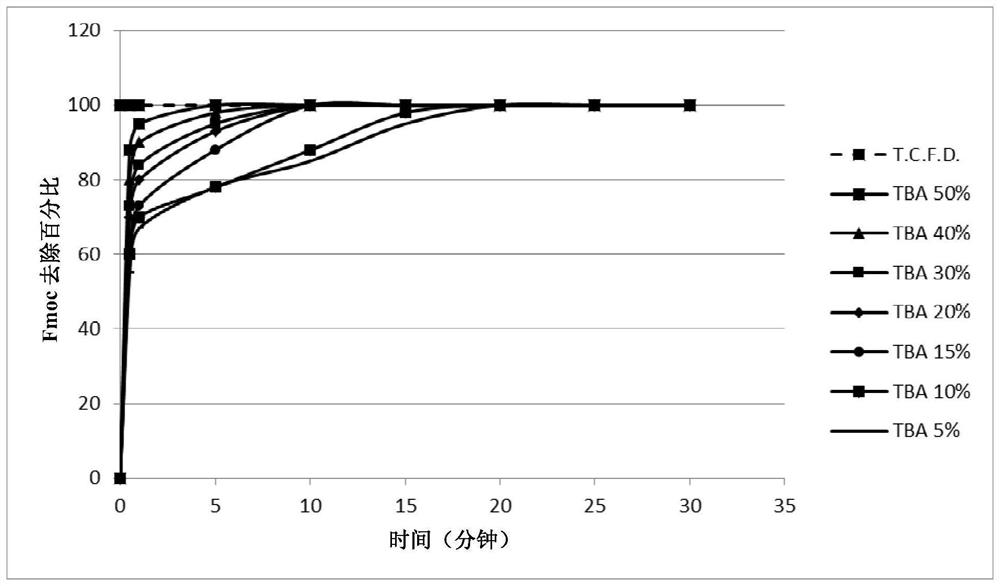
[0239] Example 2: General procedure for the study of Fmoc deprotection kinetics: screening of piperidine, TBA, N-methylpiperazine and morpholine
[0240] 10 mg Fmoc-protected linkamide resin (Fmoc-Phe(p-NO 2 ) Linkamide resin, Fmoc-Linkamide resin or Fmoc-Ser(TbU)-Linkamide resin) were swelled in DMF for 15 minutes and the amine of choice was added to the suspension so that in the final 1 ml deprotected The desired concentration (20% piperidine, 30% TBA, 5% N-methylpiperazine or 50% morpholine) was achieved in the total volume of the mixture. The reaction mixture was stirred at room temperature and samples (10 μL) of the solution were taken after 20 minutes, 1 hour 40 minutes and 20 hours. Samples were diluted with 990 μL DMF in 1 cm quartz tubes. The absorbance is measured at 301nm, and by the formula
[0241] L=(A 301 ×V×d) / (K×w×M) to calculate loading
[0242] Where L is the resin loading, A 301 is the absorbance at 301nm, V is the volume of the lysis solution, K is t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com