Preparation method of pitavastatin calcium oxide impurity
A technology for pitavastatin calcium and oxidized impurities is applied in the field of preparation of pitavastatin calcium oxidized impurities, which can solve the problems of low yield and the like, and achieve the effects of simple preparation method, improved quality control and medication safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
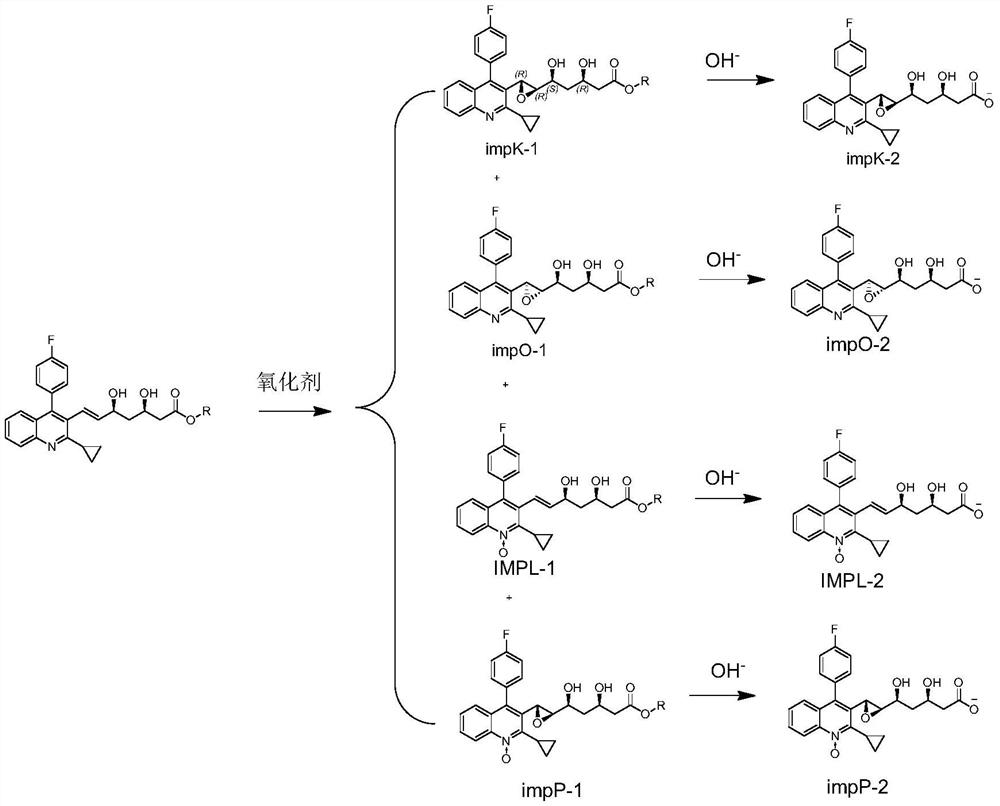
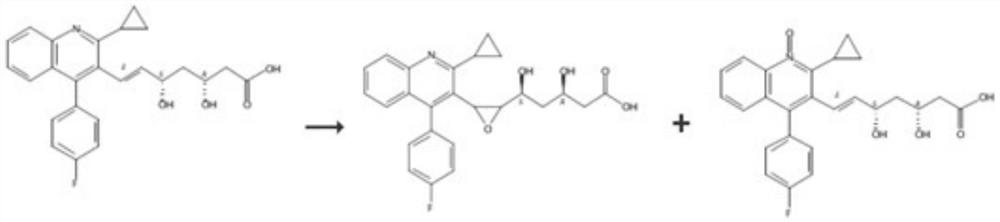
[0039] Embodiment 1: Preparation of ImpK-1, ImpL-1, ImpO-1, ImpP-1
[0040] Take 10 grams of pitavastatin ester, dissolve it in 50 mL of organic solvent, stir at a certain temperature, add dropwise an organic solvent solution of 10% mass concentration of an oxidizing agent within 1 hour, and stir at room temperature for 4 hours, add sodium bicarbonate solution Quenched, the oil phase was separated and washed once more with sodium bicarbonate, spin-dried to obtain the crude product, and the pitavastatin ester was detected by LCMS. 494.35 (ImpO-1+ImpK-1) were 18.62%, 14.08%, 47.26%, respectively. ImpK-1, ImpL-1, ImpO-1, impP-1 were obtained by preparative chromatographic separation.
[0041] The specific results are shown in Table 1 below:
[0042] Table 1
[0043]
Embodiment 2
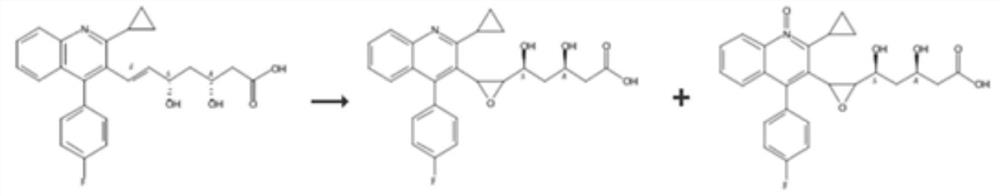
[0044] Embodiment 2: Preparation of ImpK-2, ImpL-2, ImpO-2, ImpP-2
[0045] Take 100 mg each of impK-1, impL-1, impO-1, and impP-1, add 1% alkaline aqueous solution, stir overnight at 20-25 degrees, wash the water phase with EA, and freeze-dry the water phase to obtain ImpK-2, ImpL-2, ImpO-2, ImpP-2.
[0046] The specific results of each program are shown in Table 2 below:
[0047] Table 2
[0048]
[0049] impK-2:
[0050] 1H NMR (400MHz, DMSO-d6) δ7.881 (d, J = 8.0Hz, 1H), 7.698-7.660 (m, 1H), 7.467-7.300 (m, 6H), 4.95 (s,) 4.20 (d, J=2.4Hz, 1H), 3.67(m, 1H), 3.250-3.239(m, 1H), 2.688-2.651(m, 2H), 2.012-1.965(m, 1H), 1.801-1.631(m, 1H) ), 1.378–1.056(m,5H), 1.108-1.031(m,1H)
[0051]13C NMR(101MHz,DMSO-d6)δ176.619,163.706,,161.272,162.629,146.896,145.620,132.740,132.429,132.352,131.885,131.805,129.806,128.862,127.785,126.230,125.879,125.573,116.153,115.941, 115.852, 115.640, 67.459, 66.509, 64.398, 53.387, 43.756, 40.833, 15.154, 11.563, 10.787; m / z: [M+H+]=438.2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com