Gene therapy DNA vector
A technology of gene therapy and carrier, applied in the field of genetic engineering, can solve problems such as limited further implementation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
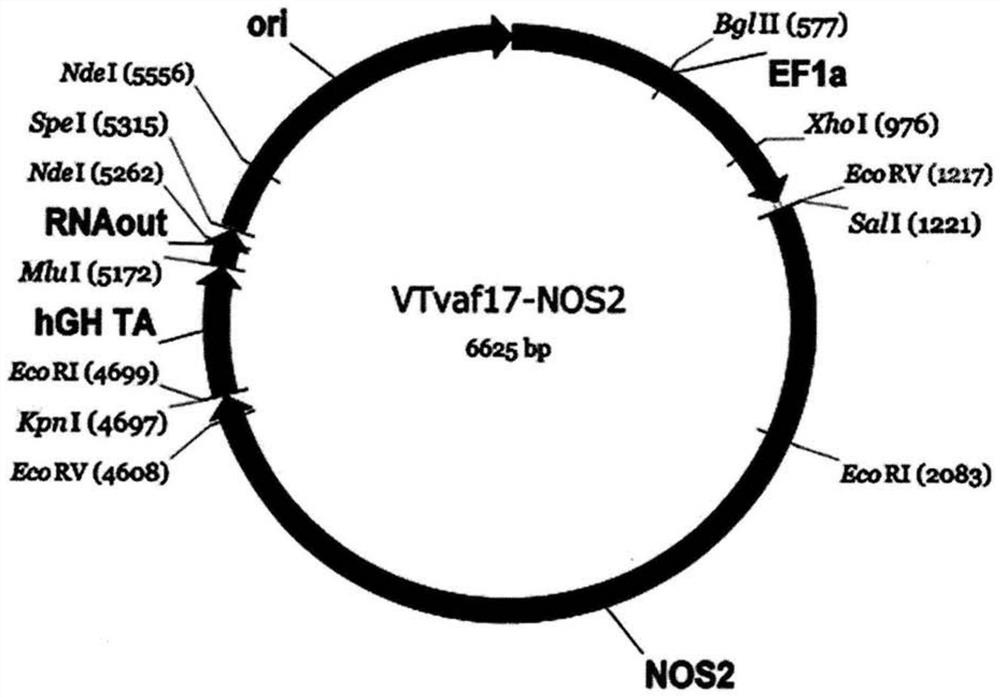
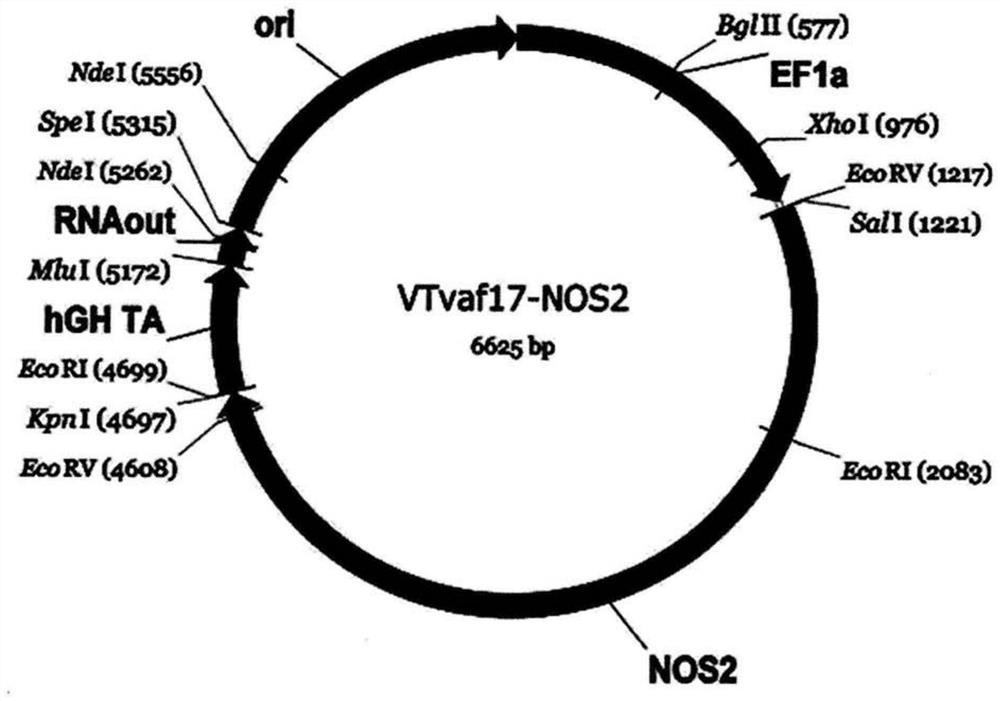
[0228] Generation of gene therapy DNA vector VTvaf17-NOS2 carrying a therapeutic gene (ie NOS2 gene).
[0229] The gene therapy DNA vector VTvaf17-NOS2 was constructed by cloning the coding region (3466bp) of the NOS2 gene into the 3165bp DNA vector VTvaf17 by SalI and KpnI restriction sites. By isolating total RNA from biological human tissue samples, followed by a reverse transcription reaction using the commercial kit Mint-2 (Evrogen, Russia), and using the following oligonucleotides as well as commercially available kits High-fidelity DNA polymerase (New England Biolabs, USA) for PCR amplification
[0230] To obtain the coding region (3466bp) of the NOS2 gene:
[0231] NOS2_F ATCGTCGACCACCATGGCCTGTCCTTGGAAATTTC,
[0232] NOS2_R CGGTACCTCCAGAGCGCTGACATCTCCAGG.
[0233] The gene therapy DNA vector VTvaf17 was constructed by integrating six fragments of DNA derived from different sources:
[0234] (a) The origin of replication was generated by PCR amplification of a regi...
Embodiment 2
[0244] Generation of gene therapy DNA vector VTvaf17-NOS3 carrying a therapeutic gene (ie NOS3 gene).
[0245] The gene therapy DNA vector VTvaf17-NOS3 was constructed by cloning the coding region (3615bp) of the NOS3 gene into the 3165bp DNA vector VTvaf17 by HindIIII and EcoRI restriction sites. By isolating total RNA from biological human tissue samples, followed by a reverse transcription reaction using the commercial kit Mint-2 (Evrogen, Russia), and using the following oligonucleotides as well as commercially available kits High-fidelity DNA polymerase (New England Biolabs, USA) was used for PCR amplification to obtain the coding region (3615bp) of the NOS3 gene:
[0246] NOS3_F GACAAGCTTCCACCATGGGCAACTTGAAGAG,
[0247] NOS3_R GGAATTCAGGGGCTGTTGGTGTCTGAGCCG; the amplified product and the DNA vector VTvaf17 were cut by restriction endonucleases HindIIII and EcoRI (New England Biolabs, USA).
[0248] This formed a 6774 bp DNA vector VTvaf17-NOS3 having the nucleotide se...
Embodiment 3
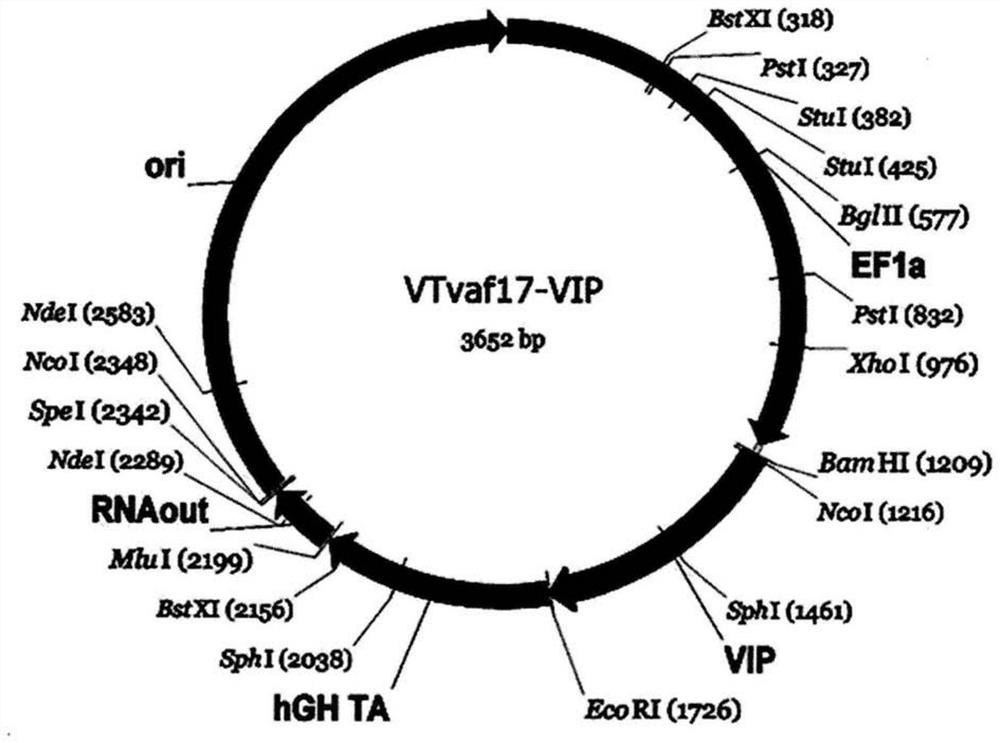
[0251] Generation of gene therapy DNA vector VTvaf17-VIP carrying a therapeutic gene (i.e. human VIP gene).
[0252] The gene therapy DNA vector VTvaf17-VIP was constructed by cloning the coding region (511bp) of the VIP gene into the 3165bp DNA vector VTvaf17 by BamHI and EcoRI restriction sites. By isolating total RNA from biological human tissue samples, followed by a reverse transcription reaction using the commercial kit Mint-2 (Evrogen, Russia), and using the following oligonucleotides as well as commercially available kits High-fidelity DNA polymerase (New England Biolabs, USA) was amplified by PCR to obtain the coding region (511bp) of the VIP gene:
[0253] VIP_F AGGATCCACCATGGACACCAGAAATAAGGCCCAG,
[0254] VIP_R GGAATTCATTTTTTCTAACTTCTTCTGGAAAG; the amplified product and the DNA vector VTvaf17 were cut by restriction endonucleases BamHI and EcoRI (New England Biolabs, USA).
[0255] This forms a 3652 bp DNA vector VTvaf17-VIP having the nucleotide sequence of SEQ ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com