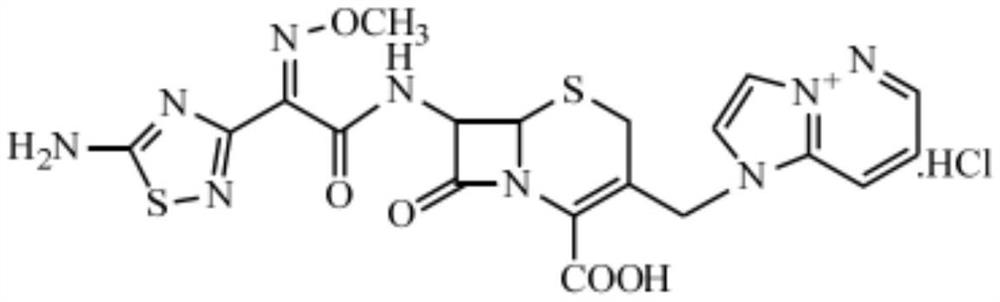
Method for purifying cefozopran hydrochloride
A technology of cefozopran hydrochloride and a purification method, which is applied in the field of preparation of cefozopran hydrochloride, can solve problems such as large amount of solvent used, difficulty in removing solvent, complex process, etc., achieves easy industrial production, solves the problem of excessive dissolution residues, and reduces drug dosage Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
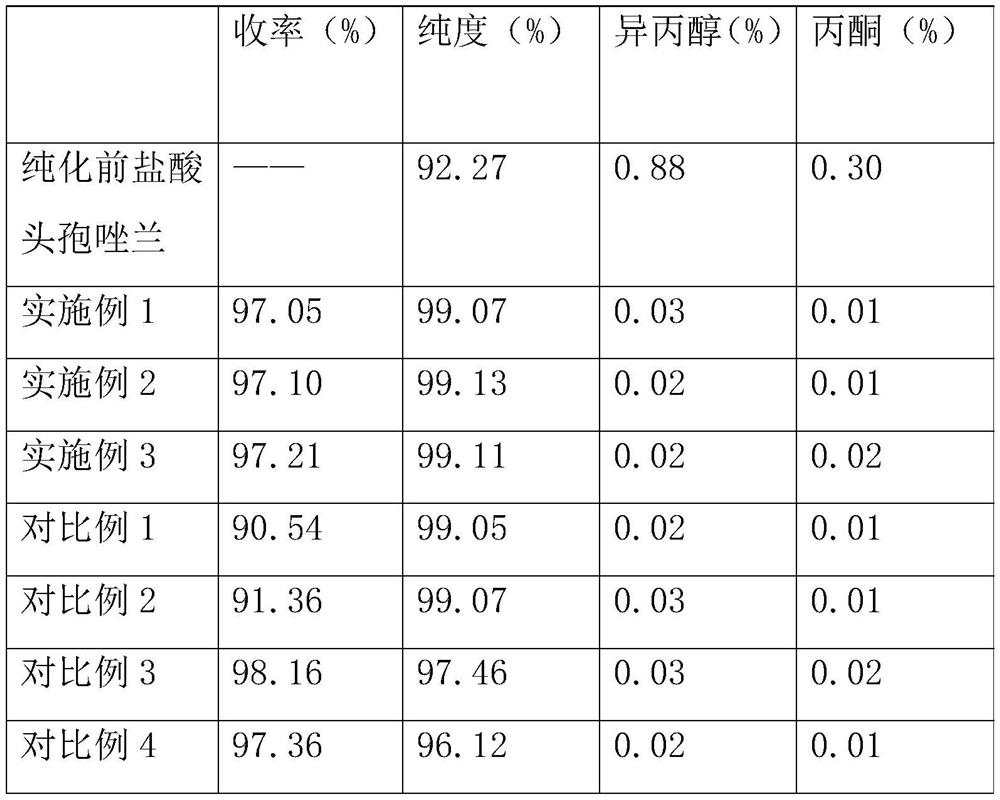
Embodiment 1
[0026] A kind of purification method of cefozopram hydrochloride, comprises the following steps successively:
[0027] (1) cefzoram hydrochloride crude product is mixed with a monobasic base in a molar ratio of 1:2.1 to generate a cefzoram sodium solution;
[0028] (2) filtering the cefozopran sodium solution to remove solid impurities, then adding hydrochloric acid to adjust the pH to neutral, adding water to separate out the cefozopran solid;
[0029] (3) reacting the cefozopram solid and hydrochloric acid separated out to make the primary product of cefozopran hydrochloride;
[0030] (4) The primary fine product of cefozopran hydrochloride is then vacuum-dried to obtain cefozopran hydrochloride.
[0031] The alkali mass concentration in the excess alkali is 30% sodium hydroxide solution. The mass concentration of the hydrochloric acid is 10%. The step (2) is operated at a temperature of 0°C. The step (3) is operated at a temperature of 0°C. The vacuum drying is to stir...
Embodiment 2
[0033] A kind of purification method of cefozopram hydrochloride, comprises the following steps successively:
[0034] (1) cefzoram hydrochloride crude product is mixed with a monobasic base in a molar ratio of 1:3.0 to generate a cefzoram sodium solution;
[0035] (2) filtering the cefozopran sodium solution to remove solid impurities, then adding hydrochloric acid to adjust the pH to neutral, adding water to separate out the cefozopran solid;
[0036] (3) reacting the cefozopram solid and hydrochloric acid separated out to make the primary product of cefozopran hydrochloride;
[0037] (4) The cefozopran hydrochloride primary fine product is vacuum-dried to obtain the cefozopran hydrochloride fine product.
[0038] The monobasic alkali in the excess alkali is 80% potassium hydroxide solution in mass concentration. The mass concentration of the hydrochloric acid is 30%. The step (2) is operated at a temperature of 5°C. The step (3) is operated at a temperature of 40°C. Th...
Embodiment 3
[0040] A kind of purification method of cefozopram hydrochloride, comprises the following steps successively:
[0041] (1) cefzoram hydrochloride crude product is mixed with a monobasic base in a mol ratio of 1:2.5 to generate a cefzoram sodium solution;
[0042] (2) filtering the cefozopran sodium solution to remove solid impurities, then adding hydrochloric acid to adjust the pH to neutral, adding water to separate out the cefozopran solid;
[0043] (3) reacting the cefozopram solid and hydrochloric acid separated out to make the primary product of cefozopran hydrochloride;
[0044] (4) The cefozopran hydrochloride primary fine product is vacuum-dried to obtain the cefozopran hydrochloride fine product.
[0045] The monobasic base in the excess base is 50% potassium hydroxide solution. The mass concentration of the hydrochloric acid is 20%. The step (2) is operated at a temperature of 2°C. The step (3) is operated at a temperature of 20°C. The vacuum drying is to stir a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com