2-aryl-4-(1H-pyrazol-3-yl)pyridine LSD1/HDAC double-target inhibitor
A technology of pyridines and aryls, applied in the field of medicinal chemistry, to achieve good selectivity, facilitate popularization and application, and good in vitro anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
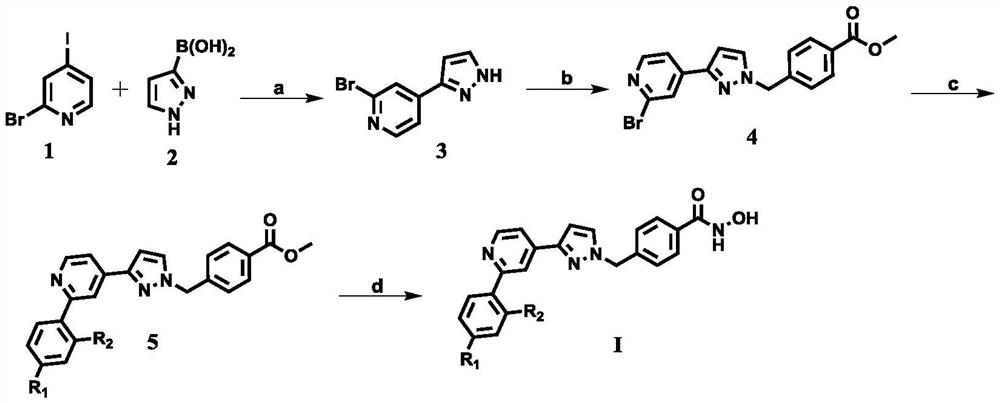
[0023] Example 1 Synthesis of 2-bromo-4-(1H pyrazol-3-yl)pyridine (3)
[0024]
[0025] Add compound 2-bromo-4-iodopyridine (1212.1mg, 6.0mmol), toluene (20mL), ethanol (25mL), H 2 O (7.5mL), K 2 CO 3 (1.68g, 11.1mmol), Pd(PPh 3 ) 4(694.0mg, 0.6mmol) and pyrazole-3-boronic acid (1451.9mg, 7.2mmol), stirred and reacted at 92°C for 4 hours under nitrogen protection. After the reaction, the reaction system was cooled to room temperature, and then added to the reaction system Extract with water and ethyl acetate, combine the ethyl acetate layers, wash with water and saturated NaCl aqueous solution respectively, dry over anhydrous sodium sulfate, filter, concentrate the filtrate under reduced pressure, and separate and purify the concentrate by silica gel column chromatography (petroleum ether: acetone = 5:1) to obtain compound 3a (850.4mg), white solid, yield: 65.5%, Mp: 140-141℃. 1 H NMR (400MHz, DMSO-d 6 )δ13.36(s,1H),8.39(d,1H,J=5.2Hz),8.03(d,1H,J=1.6Hz),7.88(s,1H),7.8...
Embodiment 2
[0026] Example 2 Synthesis of methyl 4-((3-(2-bromo-pyridin-4-yl)-1H-pyrazol-1-yl)methyl)benzoate (4)
[0027] Add compound 3 (1244.4mg, 6.0mmol), methyl 4-bromomethylbenzoate (1451.9mg, 7.2mmol) and cesium carbonate (2932.2mg, 9.0mmol) in a 50ml two-necked flask, add DMF (8mL), Stir the reaction at room temperature under nitrogen protection for 5 h. Then add water and ethyl acetate to the reaction system for extraction, combine the ethyl acetate layers, wash with water and saturated NaCl aqueous solution, dry over anhydrous sodium sulfate, filter, and concentrate the filtrate under reduced pressure. Separation and purification by silica gel column chromatography (petroleum ether: acetone = 2:1) gave compound 4 (1755.4 mg), a white solid, yield: 78.6%, Mp: 127-128°C. 1 H NMR (400MHz, CDCl 3 )δ8.36 (d, 1H, J = 5.2Hz), 8.03 (d, 2H, J = 8.0Hz), 7.90 (d, 1H, J = 1.2Hz), 7.64 (dd, 1H, J 1 =1.6Hz,J 1 =5.2Hz),7.47(d,1H,J=2.4Hz),7.29(d,2H,J=8.4Hz),6.69(d,1H,J=2.4Hz),5.43(s,2H),3....
Embodiment 3
[0028] Example 3 Synthesis of 4-((3-(2-(4-methylphenyl)pyridin-4-yl)-1H-pyrazol-1-yl)methyl)benzoate (5a)
[0029]
[0030] In a 50 ml two-neck round bottom flask, add compound 4 (521.1 mg, 1.4 mmol), toluene (5 mL), ethanol (5 mL), H 2 O (1.3mL), K 2 CO 3 (359.3mg, 2.6mmol), Pd(PPh 3 ) 4 (162.0mg, 0.14mmol) and 4-methylphenylboronic acid (231.1mg, 1.7mmol), under nitrogen protection, stirred at 92°C for 4 hours. After the reaction, the reaction system was cooled to room temperature, extracted with water and ethyl acetate , the ethyl acetate layers were combined, washed with water and a saturated NaCl aqueous solution, dried over anhydrous sodium sulfate, and filtered with suction, the filtrate was concentrated under reduced pressure, and the concentrate was purified by silica gel column chromatography (petroleum ether: ethyl acetate = 2:1), to obtain compound 5a (483.7mg), yield: 90.1%, white solid, Mp: 92-93°C. 1 H NMR (400MHz, CDCl 3 )δ8.68(dd,1H,J 1 =0.8Hz,J 2 =...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com