A kind of zeaxanthin dipalmitate liposome eye drops and preparation method thereof
A technology of palmitate and zeaxanthin, which is applied in liposome delivery, pharmaceutical formulations, active ingredients of esters, etc., can solve the problems of different, unclear use effects, no safety testing and efficacy verification, etc., to achieve the effect Diversification, enhanced bioavailability, enhanced ability to resist changes in external conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
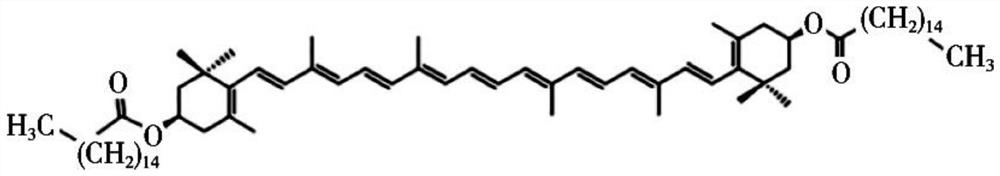
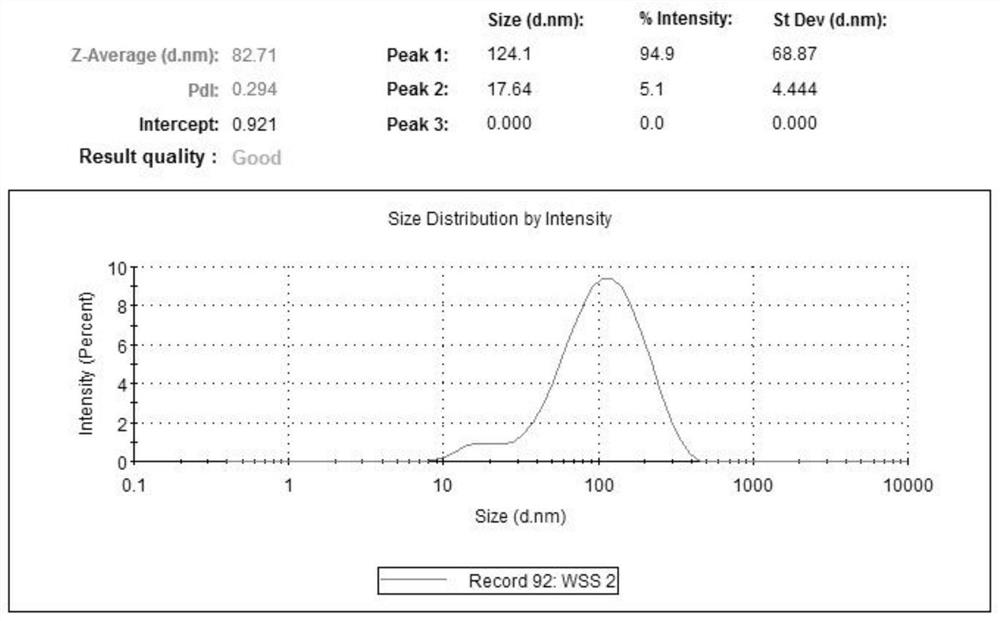
[0048] Dissolve zeaxanthin dipalmitate with a small amount of absolute ethanol (see attached for the structural formula) figure 1 ) 20mg, Tween 800.5mg and compound lecithin 400mg, steamed under reduced pressure to form a film; Measure 10mL of water for injection and preheat at 50°C; under the same conditions, add water for injection for hydration, and ultrasonicate to the solution after complete dissolution Uniform clarification, 0.22 μm filter sterilization to obtain 2 mg / mL zeaxanthin dipalmitate liposomes (attached figure 2 ), and its particle size distribution is shown in the appendix image 3 . Then, zeaxanthin dipalmitate liposomes were diluted to 1.6 mg / mL, 1.2 mg / mL, 0.8 mg / mL, 0.4 mg / mL with MEM medium containing double antibody and serum.
[0049] The compound lecithin described in the above embodiment 1 comprises egg yolk lecithin, anhydrous butter, and cholesterol, and the mass ratio thereof is 3:2:1.
Embodiment 2
[0051] Weigh the raw materials glycerol 0.05% and phenoxyethanol 0.20% by mass percentage and mix them on a magnetic stirrer, set the rotating speed to 800rpm and 35°C, dissolve and stir until uniform; Sodium phosphate 0.10%, panthenol 0.10%, vitamin B120.05%, vitamin B6 0.05% and sodium chloride 0.5% were added to the above raw materials in small amounts for several times, dissolved and stirred until uniform; the raw material zeaxanthin double palm was weighed by mass percentage Add 5.00% of ester liposome to the above-mentioned raw materials, stir until uniform to obtain a light yellow clear and transparent liquid, adjust the pH value of the liquid to 5.1-6.5 with boric acid, filter sterilize, and bottle, namely the zeaxanthin double palm Acetate liposome eye drops.
Embodiment 3
[0053] Weigh the raw material glycerol 0.05% and phenoxyethanol 0.20% by mass percentage and mix them on a magnetic stirrer, set the rotating speed to 800rpm and 35°C, dissolve and stir until uniform; weigh the raw material water for injection 88.95%, transparent Sodium phosphate 0.10%, panthenol 0.10%, vitamin B120.05%, vitamin B6 0.05% and sodium chloride 0.5% were added to the above raw materials in small amounts for several times, dissolved and stirred until uniform; the raw material zeaxanthin double palm was weighed by mass percentage Add 10.00% of ester liposome to the above-mentioned raw materials, stir until uniform to obtain a light yellow clear and transparent liquid, adjust the pH value of the medicinal liquid to 5.1-6.5 with boric acid, filter sterilize, and bottle, namely the zeaxanthin double palm Acetate liposome eye drops.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com