Preparation method of trans-4-(trans-4-alkylcyclohexyl) cyclohexanecarboxaldehyde
A technology of cyclohexane and cyclohexyl, which is applied in the field of preparation of trans-4-cyclohexane formaldehyde, can solve the problems that affect the production cost and application prospect of the product, it is difficult to remove key impurities, and the total process takes a long time. Reduced product purification process, improved atom utilization, and simple synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
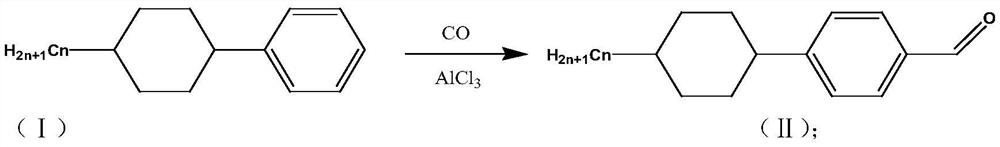
[0025] Embodiment 1, a kind of preparation method of trans-4-(trans-4-propyl cyclohexyl) cyclohexanecarbaldehyde comprises the following steps:
[0026] S1: (4-propyl-cyclohexyl)-benzene reacts with carbon monoxide in the catalyst anhydrous aluminum chloride to generate 4-(4-propyl-cyclohexyl)-benzaldehyde;
[0027]
[0028] Into a 1L reaction vessel, add 50g of (4-propyl-cyclohexyl)-benzene, 250ml of o-xylene, add 5-6 drops of concentrated hydrochloric acid, 33g of catalyst anhydrous aluminum trichloride, cool down to 0°C, under 1MPa React after feeding carbon monoxide gas under pressure. After the aeration reaction reaches the set time, vent the tail gas to normal pressure, take out the reaction solution and pour it into a beaker filled with 300mL crushed ice water, stir to make it hydrolyzed. The compound 4-(4-propyl-cyclohexyl)-benzaldehyde 54g was obtained through liquid separation, water washing, drying and distillation;
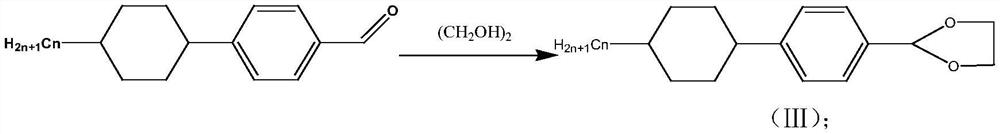
[0029] S2: 4-(4-propyl-cyclohexyl)-benzalde...
Embodiment 2
[0042] Embodiment 2, a kind of preparation method of trans-4-(trans-4-butylcyclohexyl) cyclohexanecarbaldehyde comprises the following steps:
[0043] S1: (4-butyl-cyclohexyl)-benzene reacts with carbon monoxide in the catalyst anhydrous aluminum chloride to generate 4-(4-butyl-cyclohexyl)-benzaldehyde;
[0044]
[0045] Into a 1L reaction vessel, add (4-butyl-cyclohexyl)-benzene 53.5g, o-xylene 250ml, add 5-6 drops of concentrated hydrochloric acid, catalyst anhydrous aluminum trichloride 33g, cool to 0°C, React after feeding carbon monoxide gas under 1MPa pressure. After the aeration reaction reaches the set time, vent the tail gas to normal pressure, take out the reaction solution and pour it into a beaker filled with 300mL crushed ice water, stir to make it hydrolyzed. The compound 4-(4-butyl-cyclohexyl)-benzaldehyde 57.3g was obtained through liquid separation, water washing, drying and distillation;
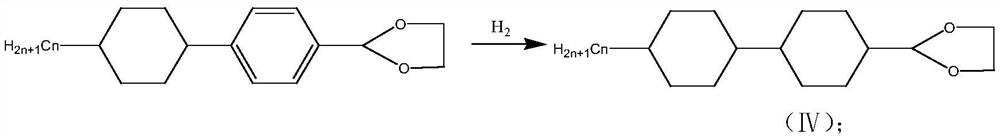
[0046] S2: 4-(4-butyl-cyclohexyl)-benzaldehyde reacts with ethyle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com