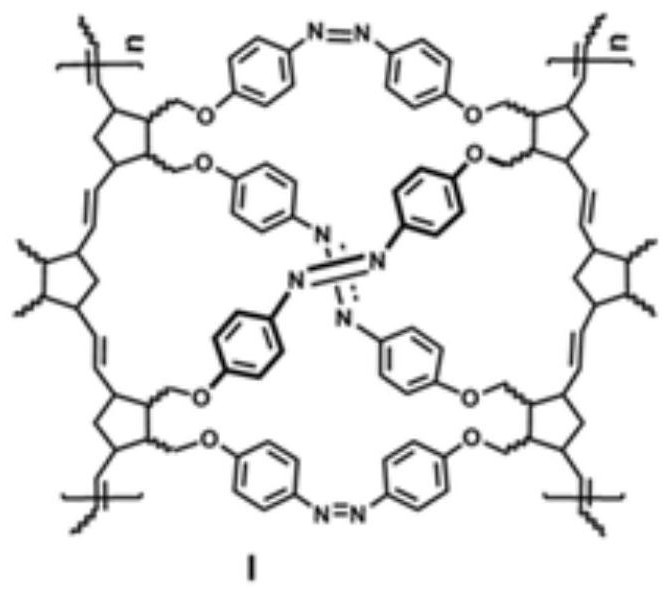
A kind of polynorbornene porous material containing azobenzene structure and preparation method thereof
A technology of polynorbornene and porous materials, applied in the field of materials, can solve the problems of small molecular weight, poor tolerance of functional groups, increased production costs, etc., and achieve the effect of mild reaction conditions and efficient methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
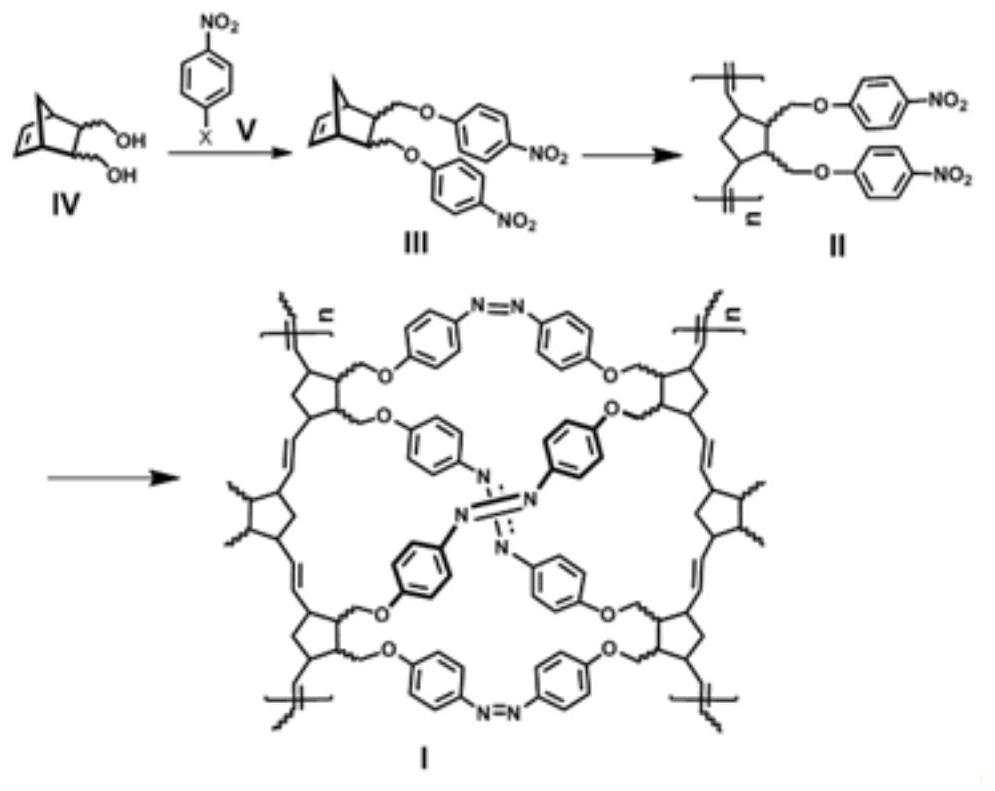
[0060] like figure 2 As shown, the flow chart of a preparation method of a polynorbornene porous material containing an azobenzene structure provided by the present invention may specifically include:
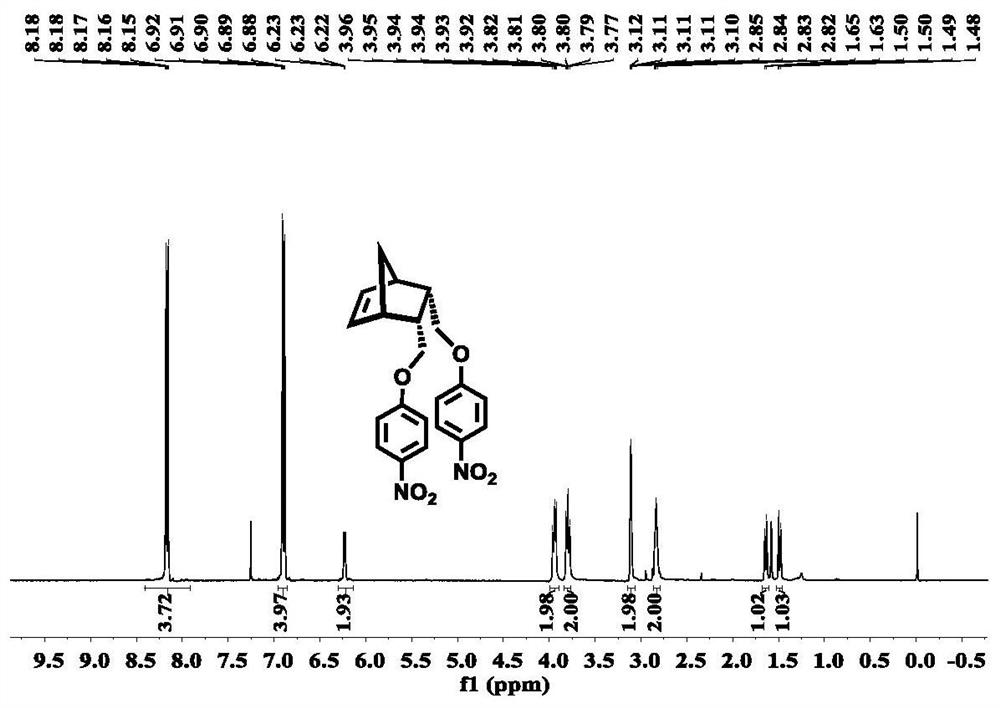
[0061] Step 1, preparation of disubstituted norbornene-p-nitrophenyl ether III: using norbornene diol shown in structural formula IV and nitrohalobenzene shown in structural formula V, to prepare disubstituted norbornene p-nitrophenyl shown in structural formula III Nitrophenyl ether;
[0062] Step 2, preparation of polynorbornene-p-nitrophenyl ether II: the disubstituted norbornene-p-nitrophenyl ether represented by the structural formula III, under the action of a ruthenium metal catalyst, undergoes an olefin ring-opening metathesis polymerization reaction to prepare the structural formula The polynorbornene p-nitrophenyl ether shown in II;
[0063] Step 3: Preparation of the azobenzene-containing polynorbornene porous material I: the polynorbornene p-nitrophenyl ether sho...
Embodiment 1
[0067] Example 1. Preparation of Endo-Configured Azobenzene-Linked Polynorbornene Porous Materials
[0068] Step 1: Preparation of Endo-configured norbornenediol with reference to related techniques
[0069] Measure DCPD (30mL, 0.35mol) in a 100mL flask with a measuring cylinder, heat up to 200°C, and obtain cyclopentadiene after distillation and condensation; Cis-1,4-butenediol (18 mL, 0.2 mol) was placed in a 250 mL sealed reaction tube, heated to 210° C. and reacted for 6 h. After cooling to room temperature, white crystals were precipitated, and the endo-configuration norbornene diol (14g, 45%) was obtained by filtration. The filtrate was dissolved in hot water (100ml, about 80°C), and the water was separated with a separatory funnel. The phase was concentrated under reduced pressure, and the oil was recrystallized to obtain endo-configuration norbornene diol (7.0 g, 23%). The overall yield was 68%).
[0070] Wherein, the DCPD is a cyclopentadiene dimer.
[0071] Step ...
Embodiment 2
[0089] Example 2. Synthesis of Endo-configured azobenzene-linked polynorbornene porous materials
[0090] Step 1: Preparation of Endo-Configured Norbornene Diols
[0091] Weigh norbornene anhydride (VI, 4.9 g, 30 mmol, 1 equiv.) with endo configuration, add it to anhydrous tetrahydrofuran (100 mL), stir to dissolve, and place it in an ice-water bath to cool; weigh out LiAlH 4 (3.6 g, 90 mmol, 3 equiv.) and slowly added to the above-mentioned mixed solution, maintained at low temperature and stirred for 0.5 hours, and slowly raised to room temperature and stirred for 12 hours. The reaction was cooled in an ice-water bath, ice-water (8 mL) was slowly added to quench the reaction, and then 10% sodium hydroxide solution (10 mL) was slowly added. After the reaction was quenched, carry out suction filtration under reduced pressure, the filter residue was washed with ethyl acetate (3×50 mL), and the filtrate was washed with anhydrous MgSO 4 Dry, concentrate and dry with a rotary ev...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com