Method for synthesizing loteprednol etabonate
A technology of loteprednol etabonate and sodium periodate, which is applied in the field of drug preparation, can solve problems such as low yield of loteprednol etabonate, waste of manpower, material resources, complicated operation, etc., to reduce production costs, reduce The generation of waste water and the effect of simple operation of the preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
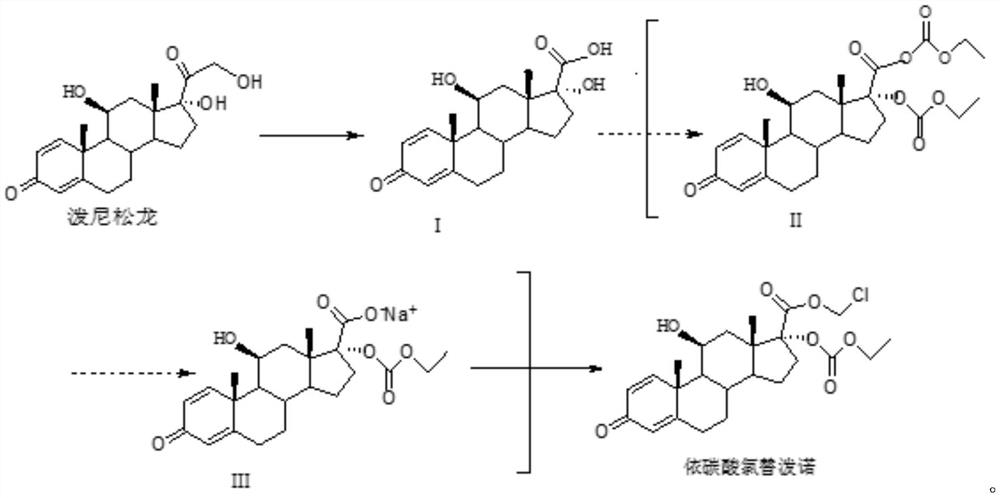
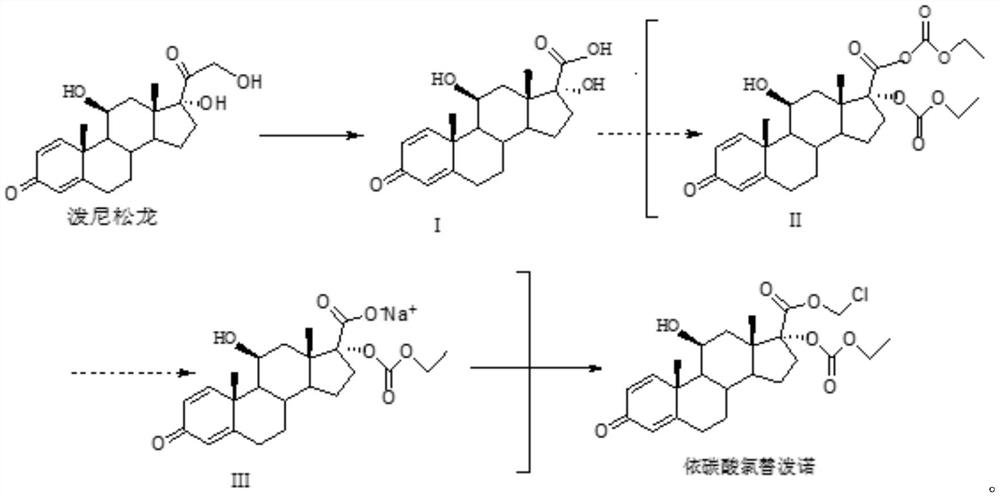
[0035] A method for synthesizing loteprednol etabonate, comprising the following steps:
[0036] S1. Mix 30g of sodium periodate with 150mL of water, stir and dissolve at 0°C to obtain a sodium periodate solution, and set aside;
[0037] Mix 20g of prednisolone with 120mL of tetrahydrofuran, add the prepared sodium periodate solution to react for 5h at 0°C, and use TLC (dichloromethane: the mass ratio of methanol is 9:1) to detect that there is almost no raw material. Tetrahydrofuran was concentrated under pressure, filtered, and the filter residue was dried for 11 hours to obtain intermediate I. The mass of intermediate I was 18.8 g, the mass yield was 94%, the HPLC content was 99.5%, and the maximum simple impurity was 0.15%;
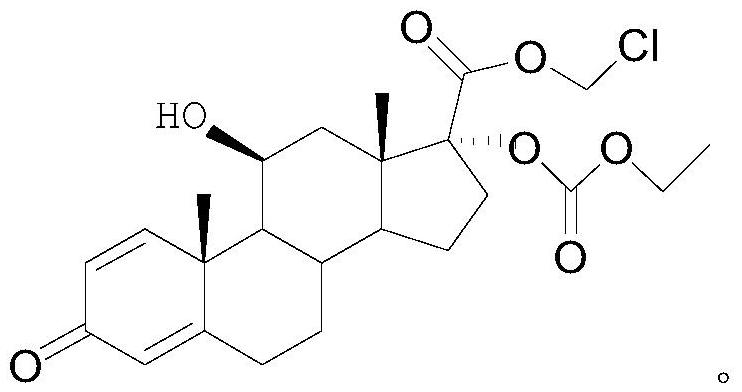
[0038] S2. Under the protection of nitrogen, mix 18g of intermediate I with 180mL of dichloromethane and 24mL of triethylamine, add 25mL of ethyl chloroformate dropwise at 5°C, and finish the dropwise addition within 30min. After reacting for 2 hours...
Embodiment 2
[0042] A method for synthesizing loteprednol etabonate, comprising the following steps:
[0043] S1. Mix 30g of sodium periodate with 150mL of water, stir and dissolve at 3°C to obtain sodium periodate solution, set aside;
[0044] Mix 20g of prednisolone with 120mL of tetrahydrofuran, add the prepared sodium periodate solution to react for 5h at 3°C, and use TLC (dichloromethane: the mass ratio of methanol is 9:1) to detect that there is almost no raw material. Tetrahydrofuran was concentrated under pressure, filtered, and the filter residue was dried for 12 hours to obtain intermediate I. The mass of intermediate I was 18.5 g, the mass yield was 92.5%, the HPLC content was 99.5%, and the maximum simplex was 0.18%;
[0045] S2. Under the protection of nitrogen, mix 18g of intermediate I with 180mL of dichloromethane and 24mL of triethylamine, add 30mL of ethyl chloroformate dropwise at 7°C, and finish the dropwise addition within 30min. After reacting for 2 hours, using TLC ...
Embodiment 3
[0049] A method for synthesizing loteprednol etabonate, comprising the following steps:
[0050] S1. Mix 30g of sodium periodate with 150mL of water, stir and dissolve at 5°C to obtain a sodium periodate solution, set aside;
[0051] Mix 20g of prednisolone with 120mL of tetrahydrofuran, add the prepared sodium periodate solution at 5°C and react for 5h, when almost no raw materials are detected by TLC (the mass ratio of dichloromethane: methanol is 9:1), the Tetrahydrofuran was concentrated under pressure, filtered, and the filter residue was dried for 12 hours to obtain intermediate I. The mass of intermediate I was 18.6 g, the mass yield was 93%, the HPLC content was 99.55%, and the maximum simplex was 0.17%;
[0052] S2. Under the protection of nitrogen, mix 18g of intermediate I with 180mL of dichloromethane and 24mL of triethylamine, add 25mL of ethyl chloroformate dropwise at 10°C for reaction, and finish the dropwise addition within 30min. Reaction at ℃ for 2 hours, u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com