Sulfhydrylated hyaluronic acid polysaccharide hydrogel as well as preparation method and application thereof
A technology of hyaluronic acid polysaccharide and hyaluronic acid, which is applied in medical science, prosthesis, tissue regeneration, etc., can solve the problems of high hardness, unsuitable vitreous body substitute, and no in vitro hydrogel, etc., to improve the resistance to degradation performance, improve the effect of shock absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
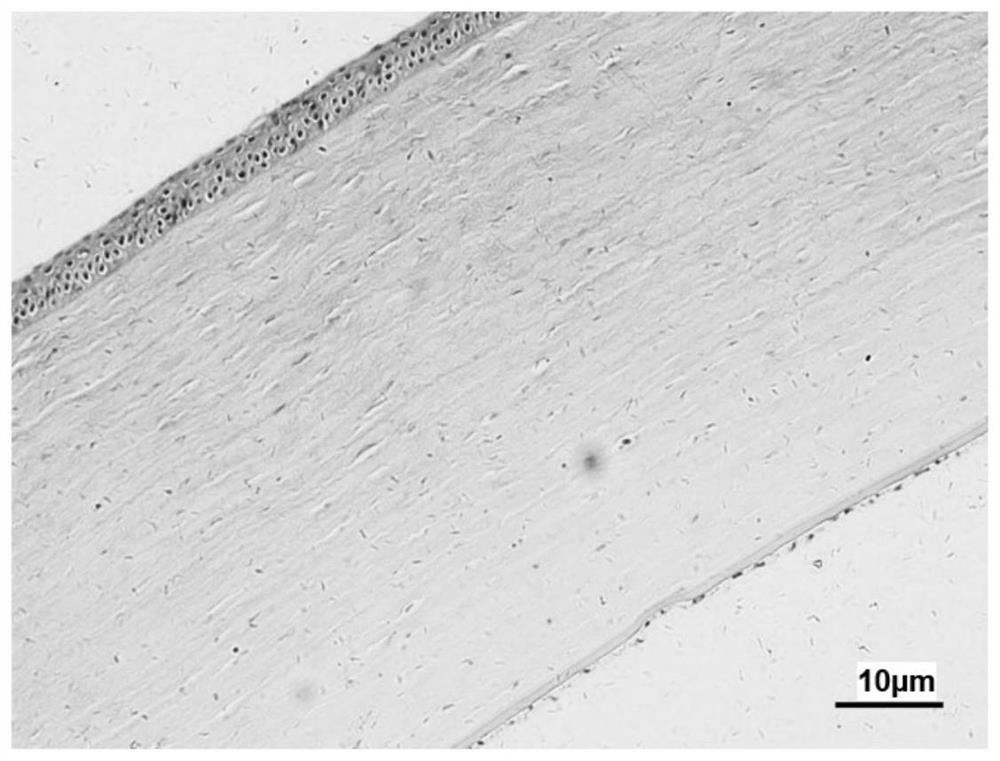
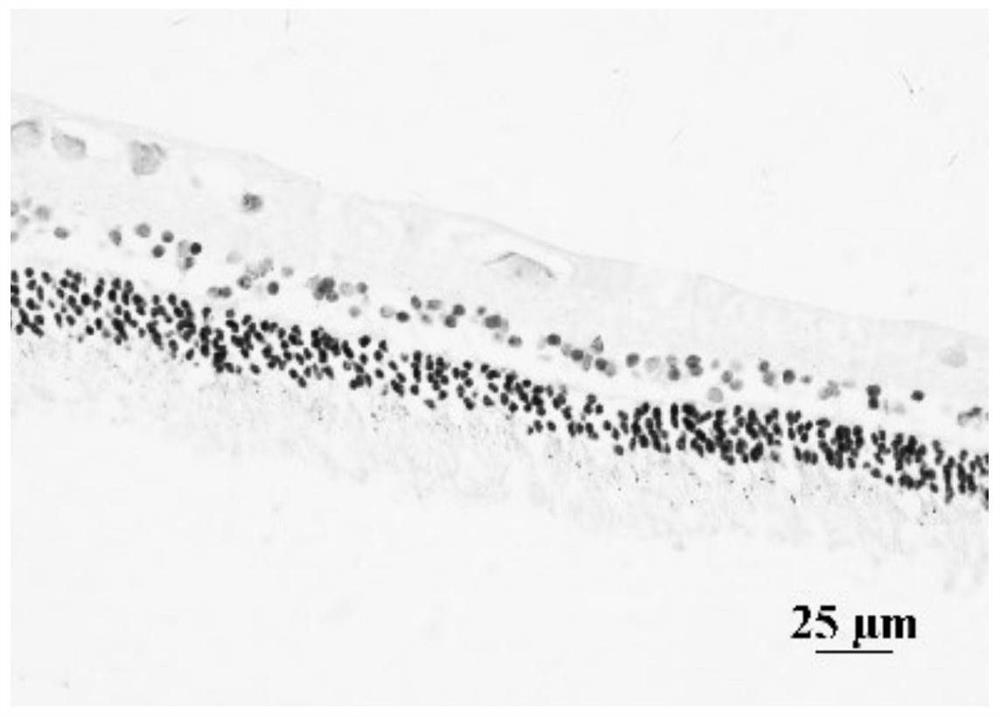
Image
Examples
Embodiment 1
[0023] Example 1: Preparation of mercaptolated hyaluronic acid derivative hydrogel (SH-HP-HA)
[0024] Weigh 2.3g hydroxypropyl hyaluronic acid (HP-HA), stir and dissolve in 100ml dimethyl sulfoxide (DMSO) to prepare a 2.3% solution, add 5.76g 1-(3-dimethylaminopropyl )-3-Ethylcarbodiimide Hydrochloride (EDC) and 3.45g N-Hydroxysuccinimide (NHS), stirring and dissolving, using 0.2mol / L NaOH solution, 0.2mol / L HCl solution to adjust the pH to 4.5, Stir at room temperature for 2 hours in the dark. Weigh 2.83 g of mercaptoethylamine hydrochloride and add it into the reaction system, adjust the pH to 4.5, and continue to react in the dark for 24 hours. After the reaction, the reactants were precipitated with ethanol, dissolved in water, washed with ethanol, precipitated with ethanol, dehydrated with ethanol, and vacuum-dried at 45°C to obtain mercaptolated hydroxypropyl hyaluronic acid (SH-HP-HA), which was sealed and stored at 4~10°C and protected from light . In this reaction...
Embodiment 2
[0026] Example 2: Preparation of mercaptolated hydroxyethyl hyaluronic acid derivative (SH-HE-HA) hydrogel
[0027] Weigh 2.35g of hydroxyethyl hyaluronic acid (HE-HA), stir and dissolve in 100ml of deionized water to prepare a 2.4% solution, add 3.84g of EDC and 2.3g of NHS, stir to dissolve, adjust the pH to 4.5, and keep away from room temperature. Light stirring for 2h. Weigh 2.36 g of cysteine hydrochloride and add it into the reaction system, adjust the pH to 4.5, and continue to react in the dark for 24 hours. After the reaction is over, put the reactant into a dialysis bag, dialyze with an acidic aqueous solution adjusted to pH 4.5 in the dark, freeze-dry after dialysis to obtain mercaptolated hydroxyethyl hyaluronic acid (SH-HE-HA), and keep away from light at low temperature Keep airtight. In this reaction, the molar ratio of HE-HA:EDC:NHS:cysteine hydrochloride was 1:4:4:3, and the measured sulfhydrylation rate was 12.26%.
[0028]Weigh 0.35 g of the prepared...
Embodiment 3
[0029] Example 3: Preparation of mercaptolated hyaluronic acid derivative hydrogel (SH-AB-HA)
[0030] Weigh 2.44g aminobutyric acid hyaluronic acid (AB-HA), stir and dissolve in 100ml deionized water to prepare a 2.4% solution, add 1.92g EDC and 1.15g NHS, stir to dissolve, adjust the pH to 5.5, and keep away from room temperature Stir lightly for 1h. Weigh 1.57 g of cysteine hydrochloride and add it into the reaction system, adjust the pH to 5.0, and continue to react in the dark for 24 hours. After the reaction is over, put the reactant into a dialysis bag, dialyze with an acidic aqueous solution adjusted to pH 4.5 in the dark, freeze-dry after dialysis to obtain mercapto-aminobutyric acid hyaluronic acid (SH-AB-HA), keep away from light at low temperature Keep airtight. In this reaction, the molar ratio of AB-HA:EDC:NHS:cysteine hydrochloride is 1:2:2:2, and the measured sulfhydrylation rate is 7.58%.
[0031] Weigh 0.45g of the prepared mercapto-aminobutyric acid h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| storage modulus | aaaaa | aaaaa |
| transmittivity | aaaaa | aaaaa |
| transmittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com