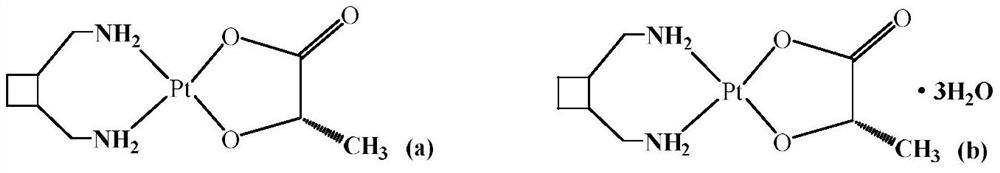
Purification method of high-purity lobaplatin trihydrate for preparing antitumor drugs
A pure lobaplatin trihydrate, lobaplatin trihydrate technology, applied in antitumor drugs, drug combinations, platinum group organic compounds, etc., can solve problems such as affecting product quality, failing to meet requirements, and introducing impurities into products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: the purification method of lobaplatin, concrete operation is as follows:
[0033] (1) Dissolve 50 g of the crude product containing lobaplatin 70.65% in 300 mL of 80°C water in a dark environment, stir for 5 minutes and then filter, place the filtrate in an environment of 0°C, stir rapidly for 3 hours, filter, and wash the filter cake with ice water 1 time, filter dry, and air-dry at 35°C for 4 hours.
[0034] (2) Product testing
[0035] In this example, the measured product content is 98.72%, the water content is 12.07% (the theoretical water content of lobaplatin trihydrate is 11.98%), and the yield is 53.37%.
Embodiment 2
[0036] Embodiment 2: the purification method of lobaplatin, concrete operation is as follows:
[0037] (1) Dissolve 50 g of the crude product containing lobaplatin 82.76% in 150 mL of 95°C water in a dark environment, stir for 2 minutes and then filter, place the filtrate in an environment of 9°C, stir rapidly for 2 hours, filter, and wash the filter cake with ice water 1 time, filter dry, and air-dry at 45°C for 2 hours.
[0038] (2) Product testing
[0039] In this example, the measured product content is 99.03%, the water content is 12.02% (the theoretical water content of lobaplatin trihydrate is 11.98%), and the yield is 52.98%.
Embodiment 3
[0040] Embodiment 3: the purification method of lobaplatin, concrete operations are as follows:
[0041] (1) Dissolve 50 g of the crude product containing lobaplatin 82.01% in 300 mL of water at 90° C. in a dark environment, stir for 3 minutes, and then filter. Add 1500 mL of diethyl ether to the collected filtrate, place in -10°C environment, stir rapidly for 2 hours, filter, stir and wash the filter cake twice with ice water, filter dry, and blow dry at 55°C for 1 hour.
[0042] (2) Product testing
[0043] In this example, the measured product content is 99.93%, the water content is 11.92% (the theoretical water content of lobaplatin trihydrate is 11.98%), and the yield is 83.18%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com