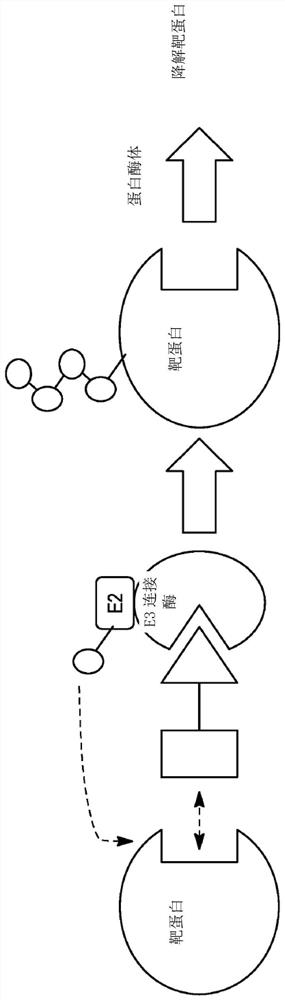
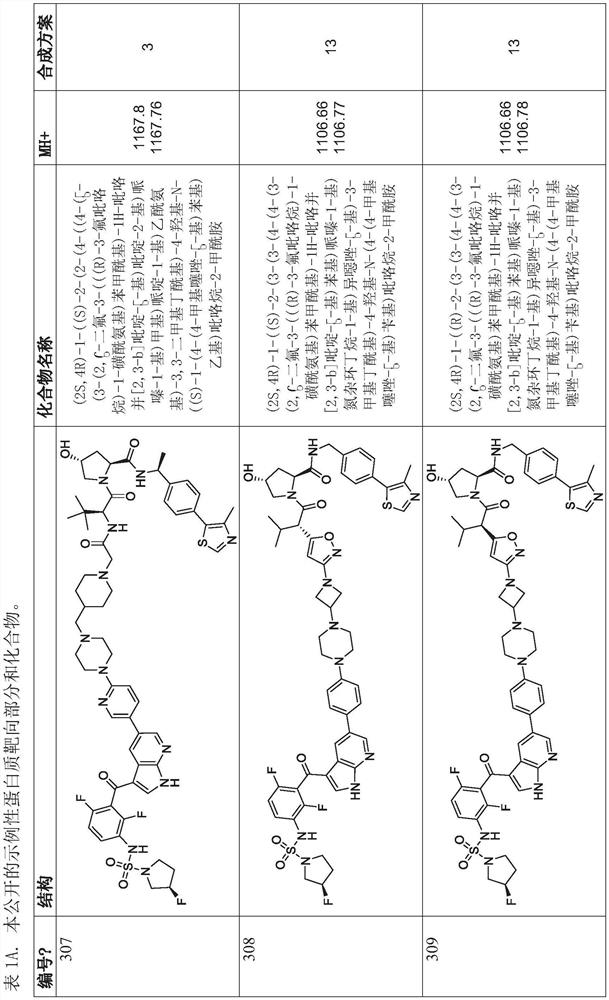
Polycyclic compounds and methods for the targeted degradation of rapidly accelerated fibrosarcoma polypeptides
A technology of bifunctional compounds and solvates, applied in the fields of peptides, drug combinations, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 20-
[1974] Example 20 - Synthesis Scheme F: Compounds 512, 513 and 516
[1975] Method F
[1976]
[1977] 4-(4-(3-(2,6-difluoro-3-(propylsulfonylamino)benzoyl)-1H-pyrrolo[2,3-b]pyridin-5-yl)phenyl) - tert-butyl piperazine-1-carboxylate (16).
[1978]
[1979] To N-[3-(5-bromo-1H-pyrrolo[2,3-b]pyridine-3-carbonyl)-2,4-difluoro-phenyl]propane-1-sulfonamide (5) (70.8 mg, 0.155mmol) in a solution of dioxane (6ml) was added 4-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolane- 2-yl)phenyl)piperazine-1-carboxylate tert-butyl ester (60.0mg, 0.155mmol), K 2 CO 3 (64.2 mg, 0.465 mmol), tricyclohexylphosphine (4.33 mg, 0.0155 mmol) and water (2 mL). The reaction mixture was then degassed under vacuum and flushed with argon (5x), to which Pd(dba) was added 2 (4.44mg, 0.00773mmol), and the reaction mixture was heated at 90°C for 3h. TLC showed a small amount of SM (Hex:AcOEt, 3:7), the reaction mixture was vacuum filtered on a pad of celite, the filtrate was poured into saturated aqu...
Embodiment 473
[3029] Step 1: Preparation of (2S,4R)-4-((tert-butyldiphenylsilyl)oxy)-2-carbamoylpyrrolidine-1-carboxylic acid tert-butyl ester
[3030]
[3031] Put (2S,4R)-2-carbamoyl-4-hydroxypyrrolidine-1-carboxylic acid tert-butyl ester (1.00g, 4.343mmol, 1 equivalent), imidazole (739.0mg, 10.855 mmol, 2.50 eq) and TBDPSCl (1.43 g, 5.203 mmol, 1.20 eq) in N,N-dimethylformamide (20 mL). The resulting mixture was stirred overnight at room temperature. Then 150 mL of water was added to quench the reaction, and the resulting mixture was extracted with ethyl acetate (250 mL×3). The organic layers were combined, washed with brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The residue was applied to a silica gel column eluting with dichloromethane / methanol (98:2). This gave 1.55 g (76.15%) of tert-butyl (2S,4R)-4-[(tert-butyldiphenylsilyl)oxy]-2-carbamoylpyrrolidine-1-carboxylate as free Color oil. LC / MS(ESI)m / z: 469.25[M+1] + .
[3032] Step 2: Pr...
Embodiment
[4454] Assay and Degradation Data
[4455] Cellular assay protocol for target protein degradation (A375 cells). A375 cells were cultured in ATCC DMEM+10% FBS in 12-well plates, and treated with the compounds shown in Tables 1-41 or 0.1% DMSO vehicle control for 16 hours. Cells were harvested in Cell Signaling Lysis Buffer (Cat. No. 9803) supplemented with Roche Protease Inhibitor Tablets (Cat. No. 11873580001), and the lysate was clarified by microcentrifugation. Proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes using the Invitrogen iBlot system. Immunoblots were performed for BRAF (Santa Cruz Cat. No. 9002), CRAF (BD Cat. No. 610151 ) and pErk (Cell Signaling Cat. No. 9106). GAPDH (catalog no.) was used as a loading control. Quantification was performed using BioRad Image Lab 5 software.
[4456] Intracellular Western Cell Assay Protocol for Target Protein Degradation (A375 Cells). A375 cells were cultured in ATCC DMEM+10% FBS in 96-well pla...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com