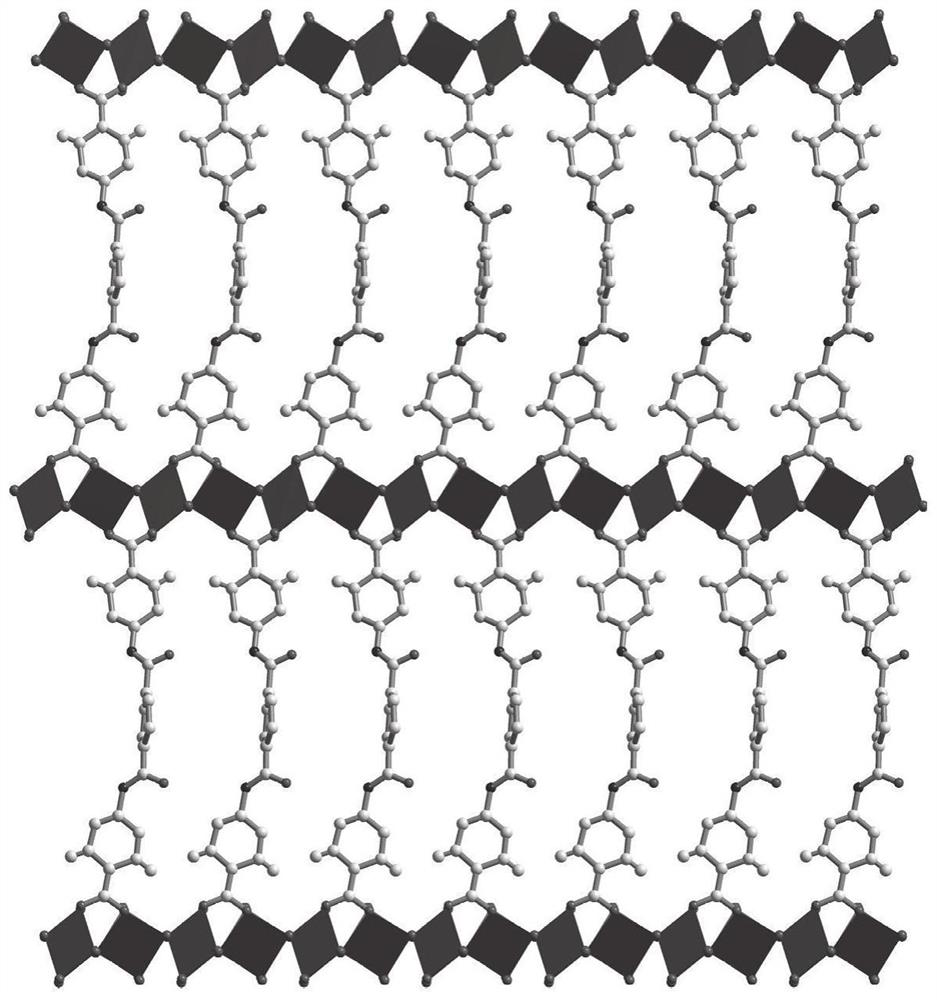
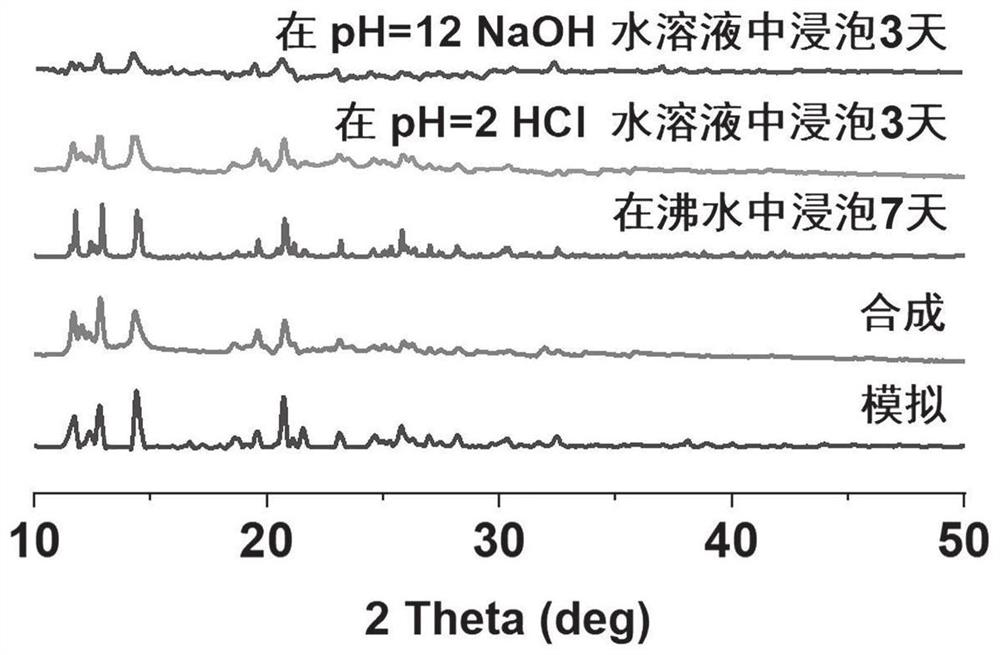
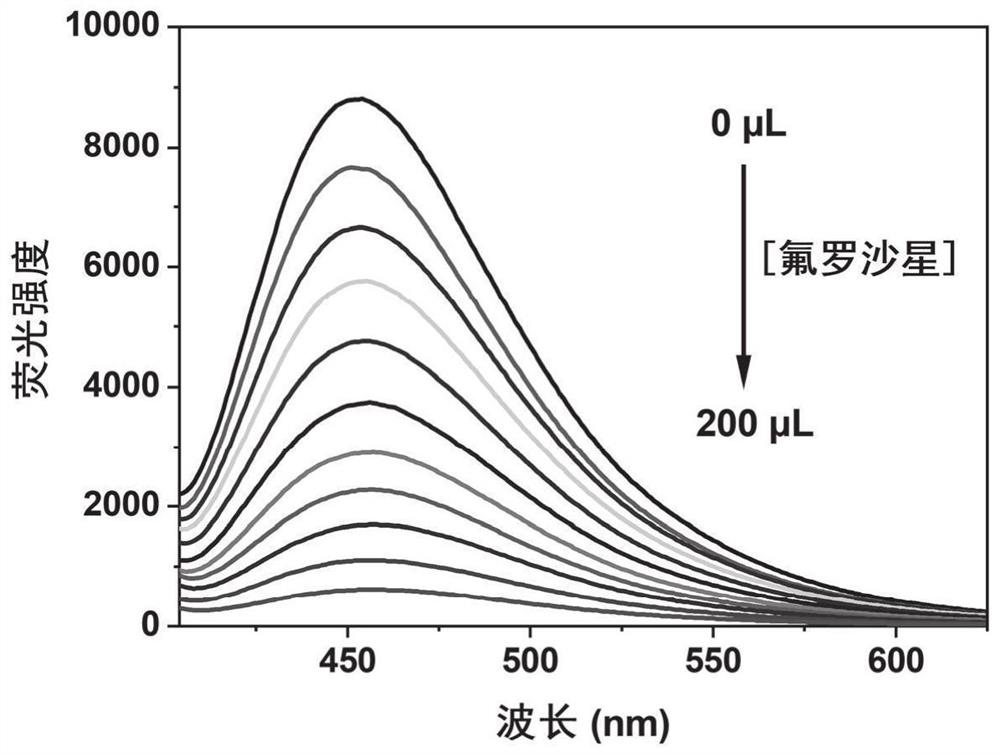
FMOF material with high water stability, preparation of FMOF material and application of FMOF material in sensing detection of fleroxacin in water
A technology of fleroxacin and stability, which is applied in the field of metal-organic framework materials, can solve the problems of being easily attacked by water molecules, ligand substitution, material decomposition, etc., and achieves high recycling rate, high selectivity, and simple preparation method Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Synthesis FMOF material: Weigh 15.4 mg of cadmium nitrate (II) tetrahydrate and 25.5 mg H 2 L is dissolved in 2.5 ml n, N'-dimethylformamide solvent, and the solution is placed in the stainless steel reactor of the inner lining polytetrafluoroethylene after ultrasonic dissolution, at 130 ° C, at 10 ° C / h is uniformly cooling to room temperature to obtain a transparent block crystal. The product was filtered, washed with N, N'-dimethylformamide, dried at room temperature, collected, and the yield was about 75%.
Embodiment 2
[0057] Synthesis FMOF material: 13.3 mg of cadmium acetate (II) dihydrate and 25.5 mg H 2 L is dissolved in 2.5 ml n, N'-dimethylformamide solvent, and the solution is placed in the stainless steel reactor of the inner lining polytetrafluoroethylene after ultrasonic dissolution, at 130 ° C, at 10 ° C / h is uniformly cooling to room temperature to obtain a transparent block crystal. The product was filtered and washed with N, N'-dimethylformamide, dried at room temperature, collected, and the yield was about 50%.
Embodiment 3
[0059] Synthesis FMOF material: Weigh 15.4 mg of cadmium nitrate (II) tetrahydrate and 25.5 mg H 2 L is dissolved in 2.5 ml n, N'-dimethylacetamide solvent, and the solution is placed in a stainless steel reaction kettle of the inner lining polytetrafluoroethylene after ultrasonic dissolution, and the constant temperature is 60 h at 130 ° C for 60 ° C. / h is uniformly cooling to room temperature to obtain a transparent block crystal. The product was filtered and washed with N, N'-dimethylacetamide, dried at room temperature, collected, and the yield was about 20%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com