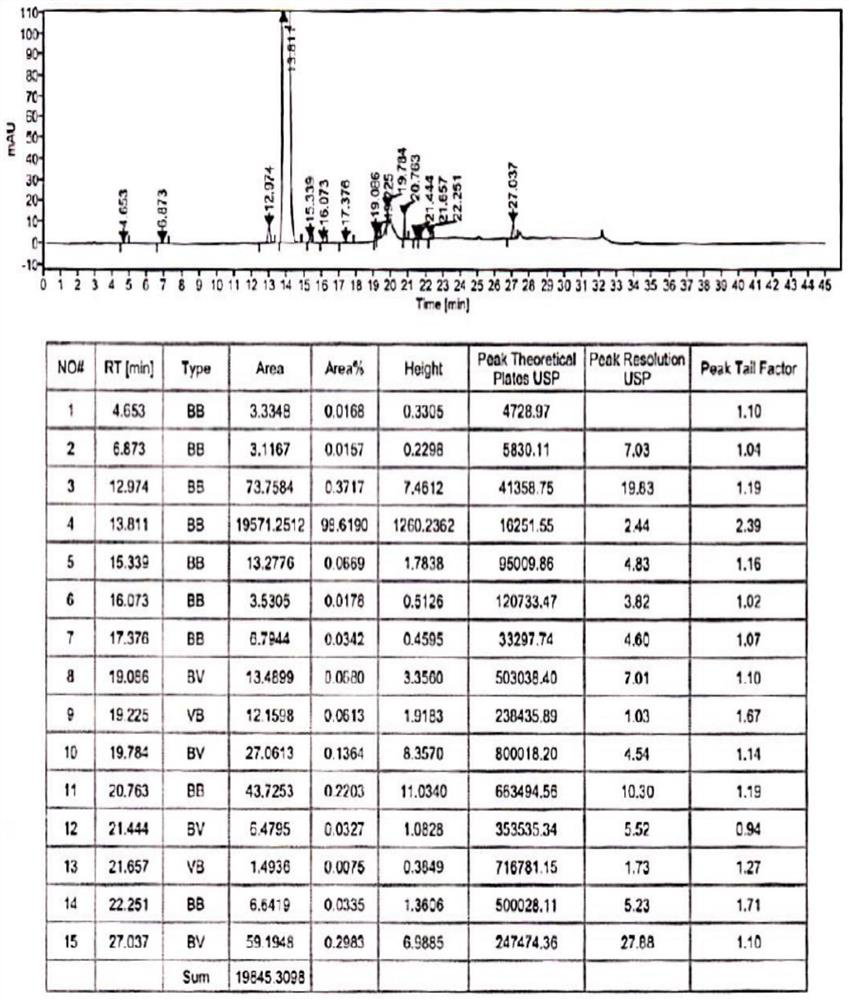
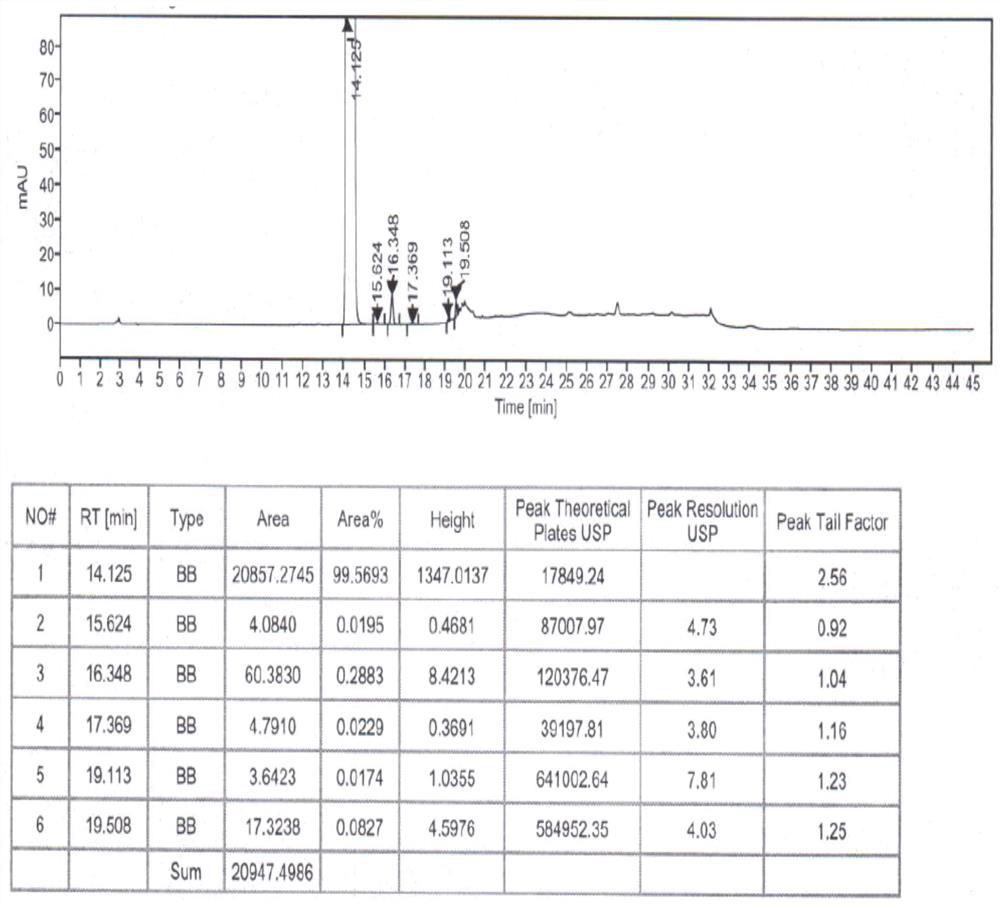
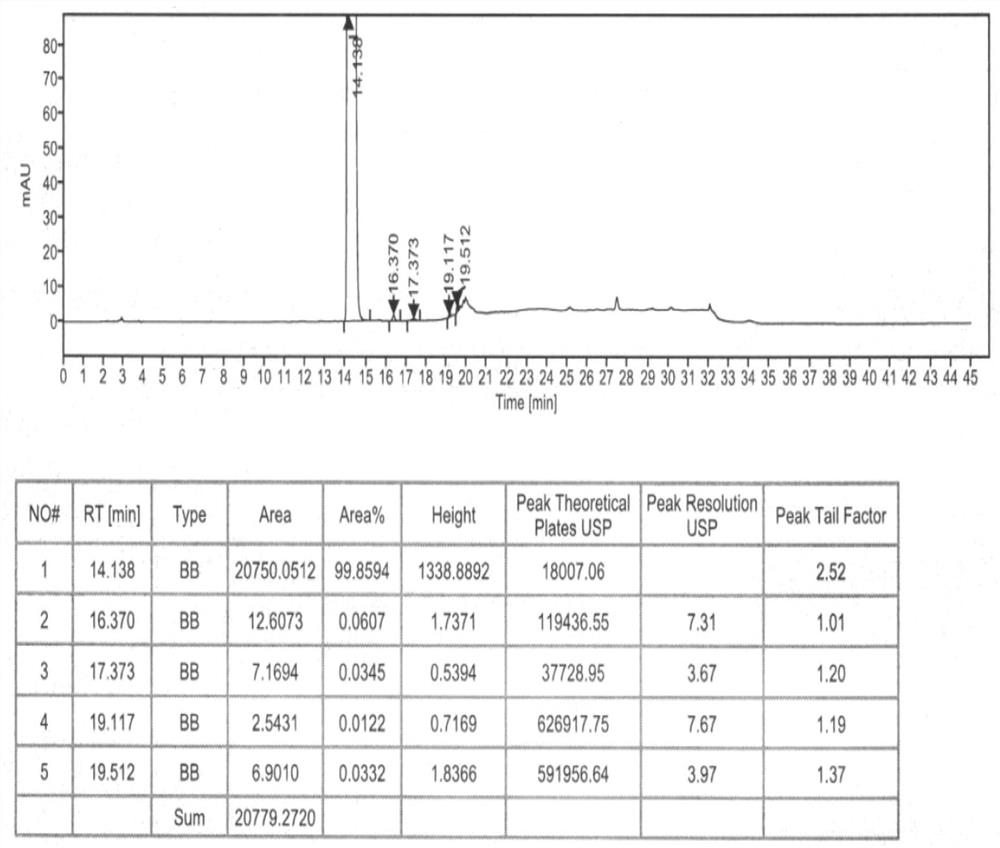
Refining method of hydroxychloroquine crude product
The technology of a crude hydroxychloroquine product and a refining method is applied to the refining field of the crude hydroxychloroquine product, and can solve the problems of being difficult to remove, low in yield, unsuitable for industrialized large-scale production, etc., and achieves the effects of low loss and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The refining of embodiment 1 hydroxychloroquine crude product
[0051] (1) Preparation of hydroxychloroquine crude product: In a 1000mL three-neck round bottom flask, add 100.02g of 4,7-dichloroquinoline, 132.07g of hydroxyquine side chain and 200ml of n-butanol, control the temperature at 130-135°C, and react for 20 Hours until the HPLC detection reaches the end of the reaction. Cool the obtained reaction solution, add 400ml of 6% sodium hydroxide aqueous solution, stir and separate the phases; the aqueous phase is extracted with 100ml of n-butanol, and the organic phases are combined and washed with 400ml of water. The organic phase was concentrated under reduced pressure at 50-65°C until no liquid dripped. Add 500ml of ethyl acetate and 5.00g of activated carbon, and stir for 1 hour at 60-70°C. Then, filter while it is hot, cool the filtrate to 30°C naturally, keep stirring for about 12 hours, and after a large amount of solids are precipitated, then lower the temp...
Embodiment 2
[0054] The refining of embodiment 2 hydroxychloroquine crude products
[0055] (1) Preparation of crude hydroxychloroquine: In a 5000mL three-neck round bottom flask, add 500.00g of 4,7-dichloroquinoline, 660.05g of hydroxyquine side chain and 1000ml of n-butanol, control the temperature at 130-135°C, and react for 20 Hours until the HPLC detection reaches the end of the reaction. Cool down the obtained reaction liquid, add 2000ml of 6% sodium hydroxide aqueous solution, stir and separate the phases; the aqueous phase is extracted with 500ml of n-butanol, and the organic phases are combined and washed with 2000ml of water. The organic phase was concentrated under reduced pressure at 50-65°C until no liquid dripped. Add 2500ml of ethyl acetate and 25.00g of activated carbon, and stir for 1 hour at 60-70°C. Then, filter while it is hot, cool the filtrate to 30°C naturally, keep stirring for about 12 hours, and after a large amount of solids are precipitated, then lower the tem...
Embodiment 3
[0058] The refining of embodiment 3 hydroxychloroquine crude products
[0059] (1) One-time refining: In a 1000mL three-neck round bottom flask, add 100g of crude hydroxychloroquine obtained in step (1) of Example 2, 1000ml of ethyl acetate, and heat up to 71°C during stirring, and wait for the crude hydroxychloroquine After completely dissolving, add 1.22g of acetic anhydride to it, keep stirring and reacting at this temperature for 1 hour, after the reaction is finished, cool down the obtained reaction solution to 30°C, keep stirring and crystallize at this temperature for 3h, wait for crystallization After finishing, filter, wash with ethyl acetate, blow dry to constant weight under the condition of 50~55 ℃, obtain hydroxychloroquine primary refined product 94.12g, the yield is 94.12%, the purity is 99.72%, the impurity III is not detected , other maximum simple impurity content is 0.19%.
[0060] (2) Secondary refining: In a 1000mL three-neck round bottom flask, add 90.02g ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com