Nicotinamide mononucleotide and protective application thereof in myocardial injury of antitumor drug
An anti-tumor drug and mononucleotide technology, which is applied in the field of nicotinamide mononucleotide and its protective application in anti-tumor drug myocardial injury, can solve the problems of research and development reports that have no therapeutic value of NMN myocardial injury, etc. Achieve the effect of improving the related characteristics of myocardial injury and low cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1. The protective effect of NMN on the injury of H9c2 cardiomyocytes caused by doxorubicin and its analogs
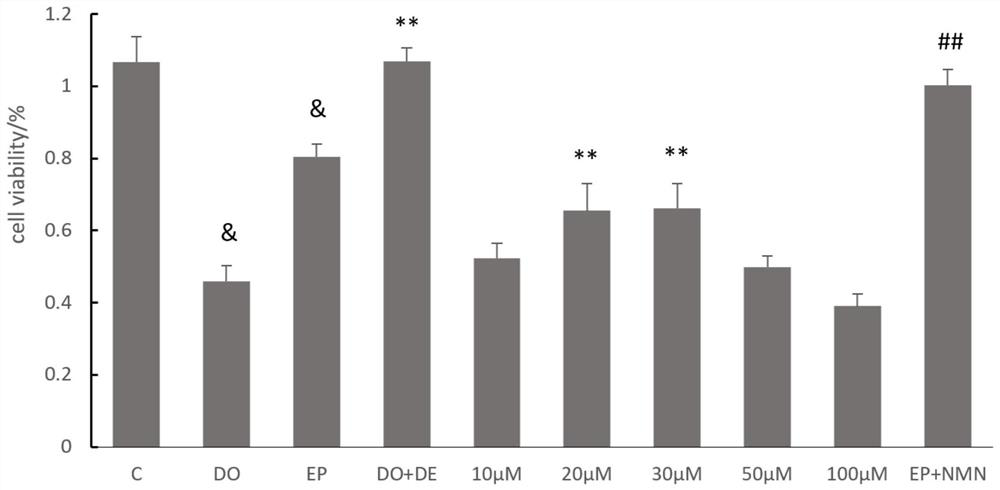
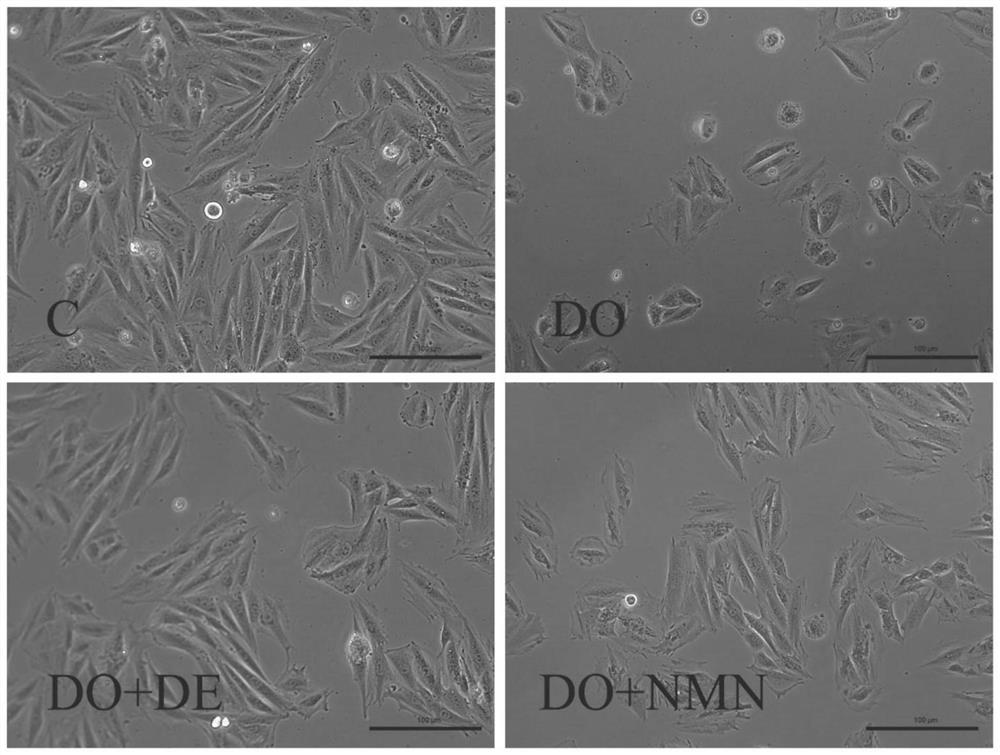
[0057] H9c2 cardiomyocytes were diluted with medium to 10 6 / ml of cell suspension, and inoculated in 96-well plates, adding 100 μL of cell suspension to each well, cultured for 24 hours, and then treated with drugs. Divided into blank control group (pure DMEM), model group 1 (5 μM DOX), model group 2 (5 μM EPI), positive control group (20 μM Dexra), 5 μM DOX+20 μM Dexra group, 5 μM DOX+NMN group (NMN 10 / 20 / 30 / 50 / 100μM), 5μM EPI+NMN group (give NMN 20μM). After culturing for 24 hours, CCK-8 cell viability was used to measure the absorbance at 450 nm using a microplate reader to calculate the cardiomyocyte activity. Cell status was observed by an inverted light microscope.
[0058] The result is as figure 1 As shown, compared with the control group cell activity of 106.7%, the 5 μM DOX model group cell activity was 46.0%, and the 5 μM EPI model group ...
Embodiment 2
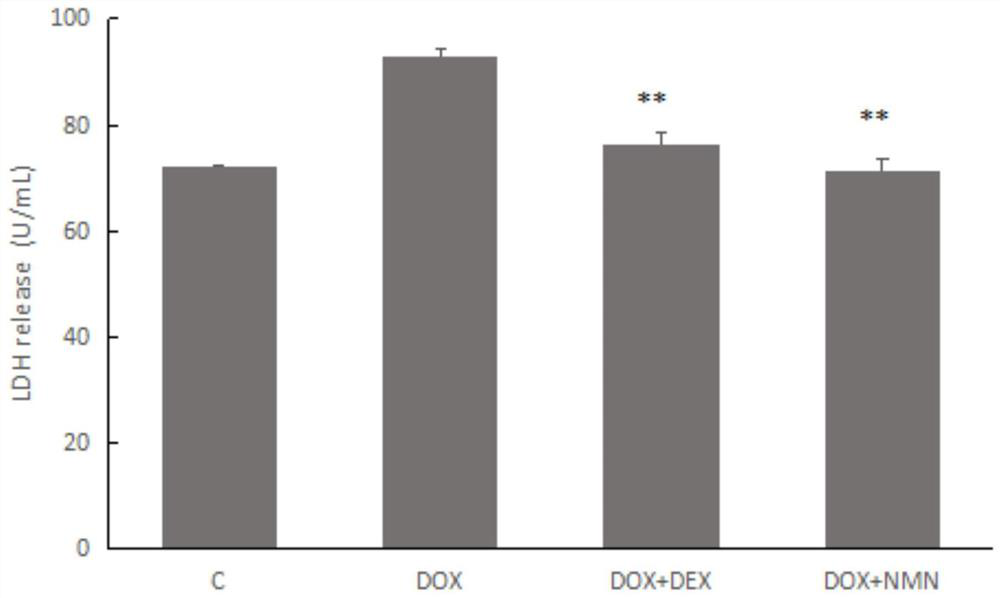
[0060] Example 2, NMN reduces the impact of doxorubicin H9c2 cardiomyocyte lactate dehydrogenase (LDH) leakage
[0061] Each group (n=4) takes 10 for each sample 6 Cells were fully lysed, and the content of LDH in the cells was detected by the pyruvate method. The LDH in the cells of the control group was 100%. 1 μmol of pyruvate produced in the system was regarded as 1 unit, calculated per unit / mg protein. After culturing for 24 h, the LDH detection kit was used for determination. The results show( image 3 ) The leakage rate of LDH in different medication groups was significantly lower than that in the model group.
Embodiment 3
[0062] Example 3, NMN inhibits the influence of doxorubicin H9c2 cardiomyocyte lipid peroxidation level
[0063] Oxygen free radicals attack polyunsaturated fatty acids in biomembranes, triggering lipid peroxidation and thus generating the lipid peroxide malondialdehyde (MDA). MDA can be condensed with thiobarbituric acid (TBA) to form a red product with a maximum absorption peak at 532nm. Adopt the same treatment method as in Example 2, and after culturing for 24 hours, operate according to the instructions of the MDA detection kit. The results show( Figure 4 ), the MDA content in the myocardial injury model group was significantly higher than that in the control group, P<0.05. The DOX+NMN group was significantly lower than the model group, confirming that NMN can inhibit lipid peroxidation induced by reactive oxygen species.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com