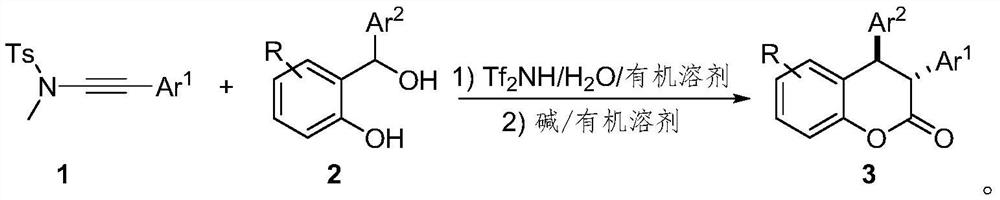
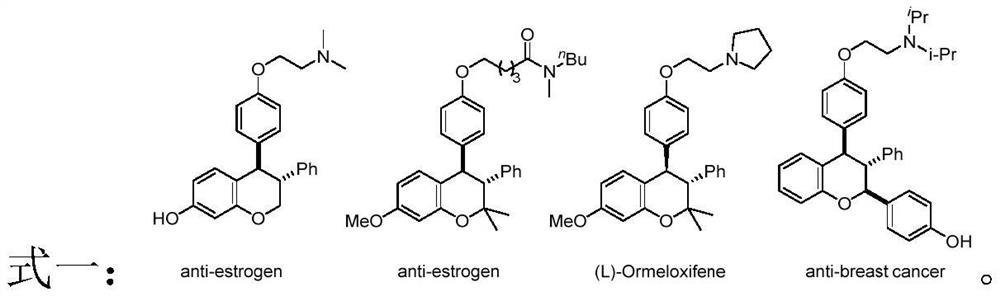
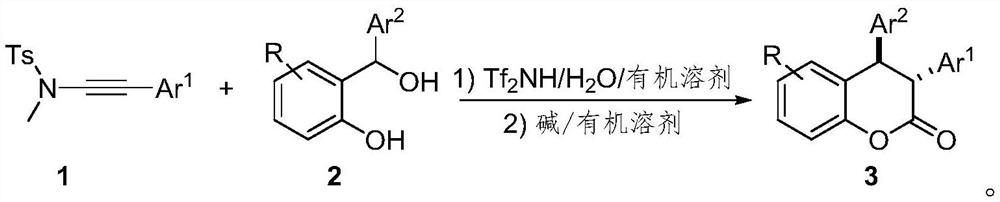
Preparation method of trans-3, 4-diaryl dihydrocoumarin compound
A technology of diaryl dihydrocoumarins and compounds, applied in organic chemistry methods, organic chemistry, etc., can solve the problems of rare reports and limitations of trans products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0025] Below in conjunction with specific embodiment, the present invention is described in further detail. In the following, unless otherwise specified, all reagents used were purchased from commercial sources without further purification. The progress of the reaction was monitored by TLC. The silica gel used is 300-400 mesh silica gel. Diastereoselectivity and related configurations based on 1 H NMR and obtained by the method of literature Org. Lett., 2018, 20, 4769.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com