A kind of fluorescent probe for detecting ethylene and its preparation method and application
A fluorescent probe and reaction technology, applied in the field of fluorescent probes, can solve the problems of poor biocompatibility and low sensitivity, and achieve the effects of good selectivity, high sensitivity, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Synthesis of embodiment 1 fluorescent probe CEP-1
[0030] N-coumarinoxyamide (21.9 mg, 0.1 mmol), dichloro(pentamethylcyclopentadienyl) rhodium (III) dimer (34.0 mg, 0.055 mmol) and potassium carbonate (13.8 mg , 0.1 mmol) was added to 2 mL of acetonitrile solvent. Under the protection of nitrogen, the mixture was stirred and reacted for 8 h at 25°C at room temperature, then filtered and washed with ether to obtain the target probe compound CEP-1 with a yield of 95%. 1 H NMR (500MHz, methanol-d 4 )δ7.83(d, J=9.3Hz, 1H), 7.20(d, J=8.2Hz, 1H), 6.90-6.69(m, 1H), 6.15(d, J=9.4Hz, 1H), 2.22( s, 3H), 1.66 (s, 15H); 13C NMR (125MHz, methanol-d 4 )δ170.5, 165.2, 164.2, 159.2, 145.7, 125.8, 113.8, 110.0, 105.1, 96.7, 21.42, 8.3; ESI-HRMS m / z: 456.0696 ([M+H] + ).
Embodiment 2
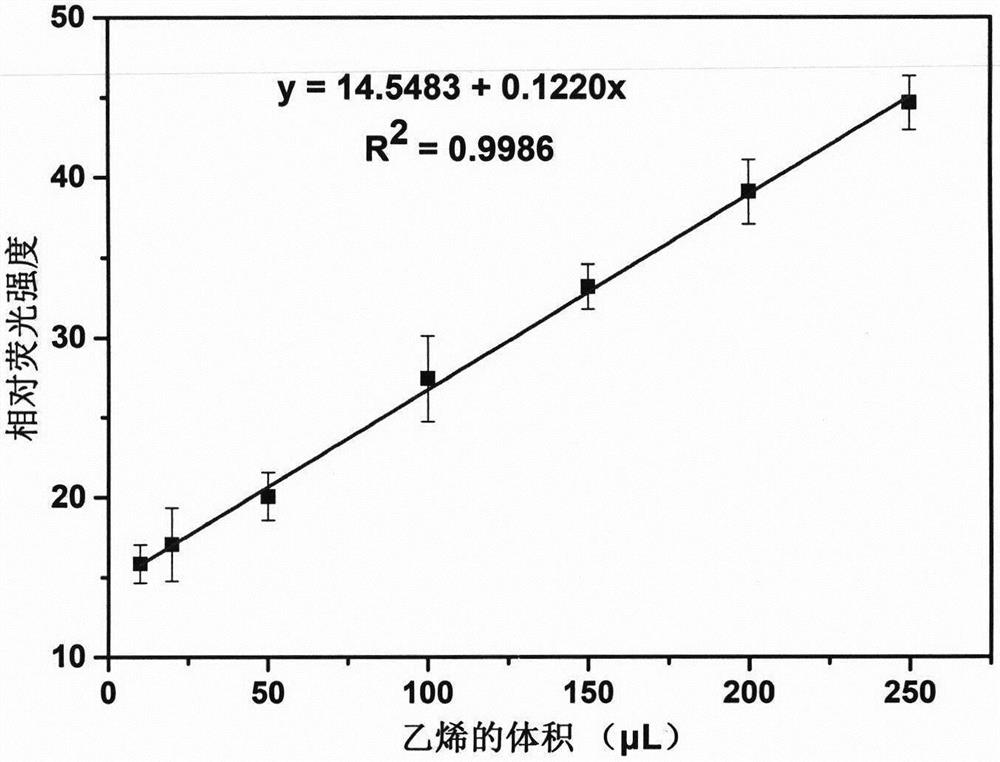
[0031] Fluorescence intensity of fluorescent probe CEP-1 under different volumes of ethylene in embodiment 2
[0032] 10, 20, 50, 100, 150, 200 and 250 μL volumes of ethylene were added to the CEP-1 probe solution (100 μM) through a gas syringe, and the fluorescence detection (λ ex =365nm,λ em =460nm), detect the fluorescence intensity in each system, and make a curve with the volume of relative fluorescence intensity-ethylene, such as figure 1 As shown, the minimum detection limit of the probe to ethylene was finally obtained as 52ppb.
Embodiment 3
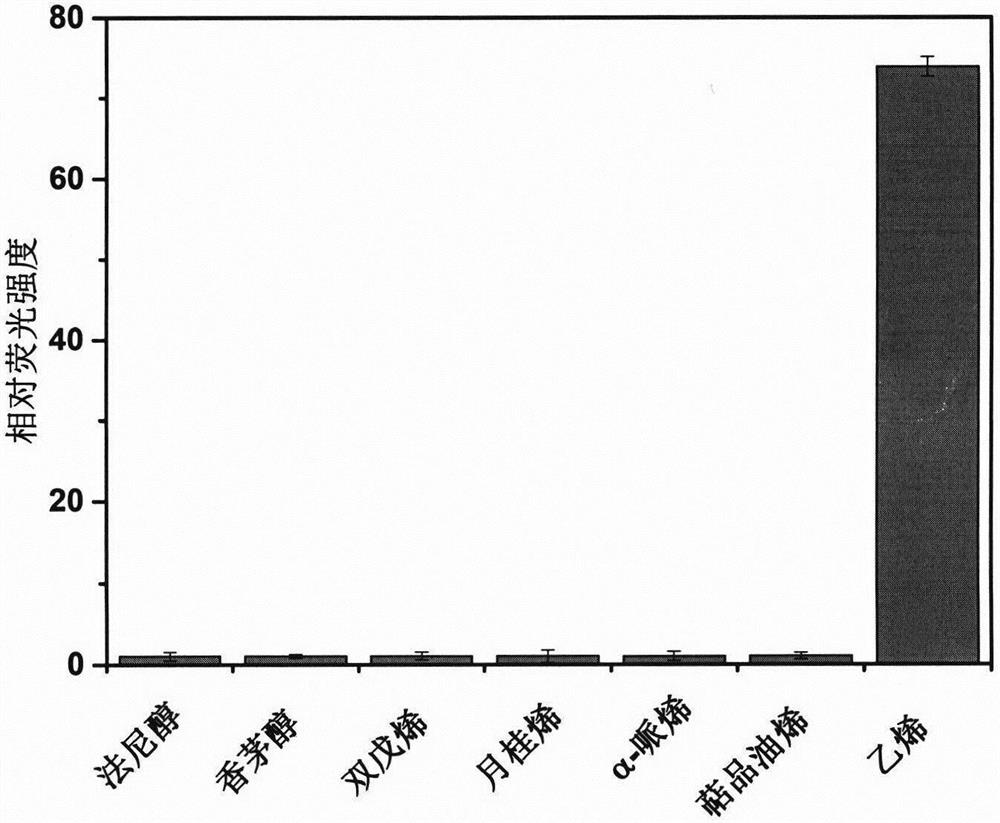
[0033] The selectivity of embodiment 3 ethylene probes
[0034] Olefins in plants or the environment may interfere with the detection of ethylene, so it is necessary to determine whether the synthetic ethylene fluorescent probe has good selectivity in recognizing ethylene.
[0035] Take 9 sample vials, add 100 μM CEP-1 fluorescent probe solution, 1 sample vial is used as blank control, and add 1 mL of ethylene (concentration is about 2 mM), 6 mM farnesol, 6 mM citronellol respectively to 9 sample vials , 6mM dipentene, 6mM myrcene, 6mMα-pinene and 6mM terpinolene, after reacting for 1min, record the fluorescence intensity of different reaction systems, the results are as follows figure 2 shown. Depend on figure 2 It can be seen that the probe solution only responds to ethylene, and the fluorescence intensity increases nearly 50 times, while the fluorescence intensity of other reaction systems does not change significantly, which shows that the fluorescent probe is highly s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com