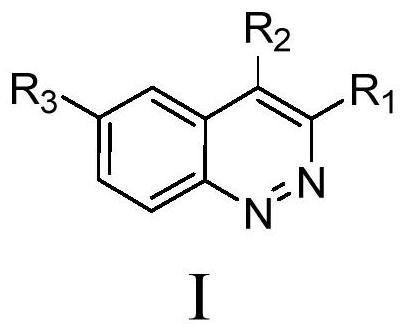
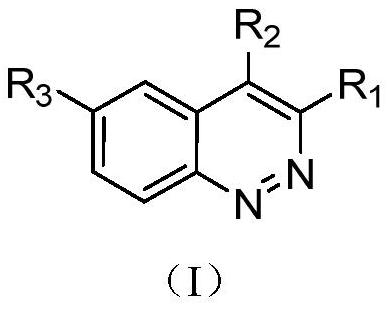
Cnoline compound PI3K kinase inhibitor as well as preparation method and application thereof in pharmacy
A technology of kinase inhibitors and compounds, applied in the field of medicinal chemistry, can solve the problems of not being able to treat PI3K kinase, not having PI3K kinase targeting, and achieving good therapeutic effect and safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
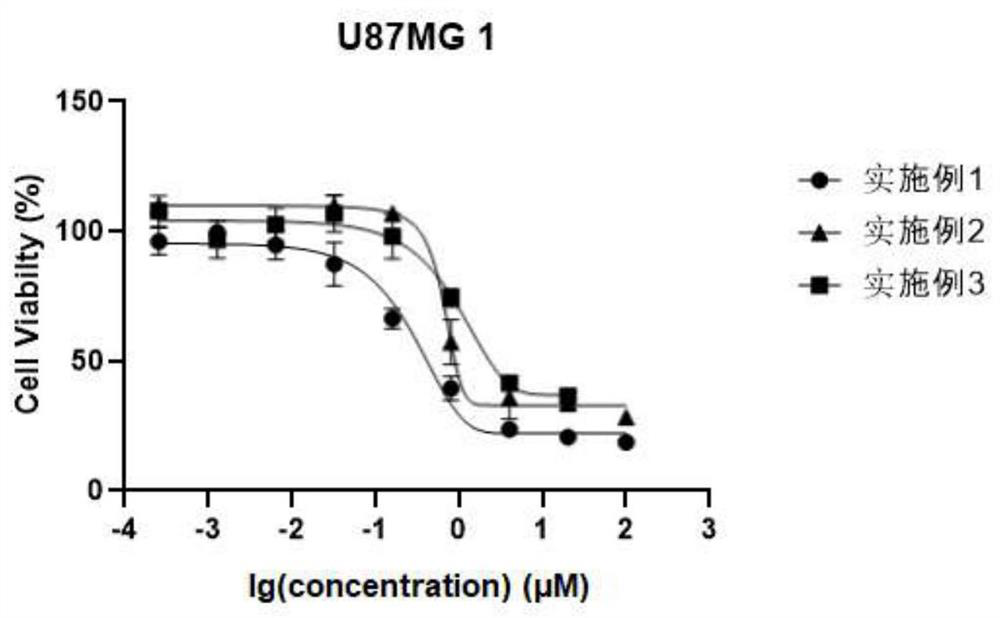
Embodiment 1
[0153] Example 1: 4-morpholino-6-(2-methoxy-3-benzenesulfonylaminopyridin-5-yl)cinnoline
[0154]
[0155] With 0.43g (1.10mmol) 2-methoxy-3-benzenesulfonylaminopyridine-5-boronic acid pinacol ester, 0.29g (1.00mmol) 4-morpholino-6-bromocinnoline, 0.35g ( 2.50mmol) anhydrous K 2 CO 3 , 0.04g (5% mol) PdCl 2 (dppf), 10mL 1,4-dioxane, 2mL water into a 100mL flask, N 2 Reflux under protection for 8h. After the completion of the reaction was confirmed by TLC, 1,4-dioxane was removed by rotary evaporation, and an appropriate amount of EA was added to mix with silica gel to pass through the column to obtain 0.25 g of 4-morpholinyl-6-(2-methoxy-3-benzenesulfonylamino pyridin-5-yl)cinnoline (49%). 1 H-NMR (400MHz, DMSO-d6): δppm 3.39 (t, J = 4.48Hz, 4H), 3.75 (s, 3H), 3.95 (t, J = 4.26Hz, 4H), 7.59 (t, J = 7.66 Hz, 2H), 7.68(t, J=7.46Hz, 1H), 7.73(d, J=7.32Hz, 2H), 7.91(s, 1H), 8.15(d, J=8.88Hz, 1H), 8.20( s,1H),8.24(s,1H),8.53(d,J=8.88Hz,1H),8.62(s,1H),9.02(s,1H),10.03(brs,...
Embodiment 2
[0156] Example 2: 4-morpholino-6-(2-chloro-3-benzenesulfonylaminopyridin-5-yl)cinnoline
[0157]
[0158] 0.43g (1.10mmol) 2-chloro-3-benzenesulfonylaminopyridine-5-boronic acid pinacol ester, 0.29g (1.00mmol) 4-morpholino-6-bromocinnoline, 0.27g (2.50mmol ) Anhydrous Na 2 CO 3 , 0.05g (6% mol) PdCl 2 (dppf), 12mL 1,4-dioxane, 3mL water into a 100mL flask, N 2 Reflux under protection for 8h. After the completion of the reaction was confirmed by TLC, 1,4-dioxane was removed by rotary evaporation, and an appropriate amount of EA was added and mixed with silica gel to pass through the column to obtain 0.36 g of 4-morpholinyl-6-(2-chloro-3-benzenesulfonylaminopyridine- 5-yl)cinnoline (75%). 1 H-NMR (400 MHz, DMSO-d6): δppm 3.48 (t, J = 4.38Hz, 4H), 3.90 (t, J = 4.24Hz, 4H), 7.59 (t, J = 7.62Hz, 2H), 7.68 (t, J=7.50Hz,1H),7.78(d,J=7.64Hz,2H),8.14(d,J=8.92Hz,1H),8.14(s,1H),8.18(s,1H),8.44 (d,J=8.56Hz,1H),8.73(s,1H),9.04(s,1H),10.71(brs,1H)
Embodiment 3
[0159] Example 3: 4-phenylamino-6-(2-methoxy-3-benzenesulfonylaminopyridin-5-yl)cinnoline
[0160]
[0161] With 0.43g (1.10mmol) 2-methoxy-3-benzenesulfonylaminopyridine-5-boronic acid pinacol ester, 0.30g (1.00mmol) 4-phenylamino-6-bromocinnoline, 0.35g ( 2.50mmol) anhydrous K 2 CO 3 , 0.04g (5% mol) PdCl 2 (dppf), 10mL 1,4-dioxane, 2mL water into a 100mL flask, N2 Reflux for 16h under protection. After the completion of the reaction was confirmed by TLC, 1,4-dioxane was removed by rotary evaporation, and an appropriate amount of EA was added to mix with silica gel to pass through the column to obtain 0.19 g of 4-phenylamino-6-(2-methoxy-3-benzenesulfonylamino pyridin-5-yl)cinnoline (39%). 1 H-NMR (400MHz, DMSO-d6): δppm 3.54(s, 3H), 7.25(t, J=7.70Hz, 1H), 7.40(d, J=7.46Hz, 2H), 7.50(t, J=7.80 Hz,2H),7.56(t,J=7.68Hz,2H),7.71(t,J=7.50Hz,1H),7.77(d,J=7.76Hz,2H),8.13(s,1H),8.19( d,J=8.88Hz,1H),8.29(s,2H),8.48(s,2H),8.78(s,1H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com