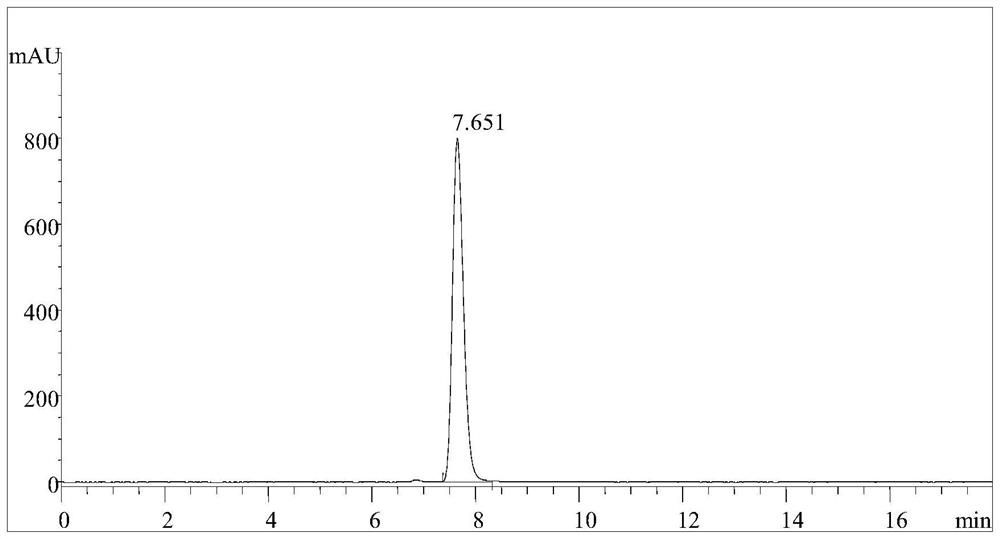
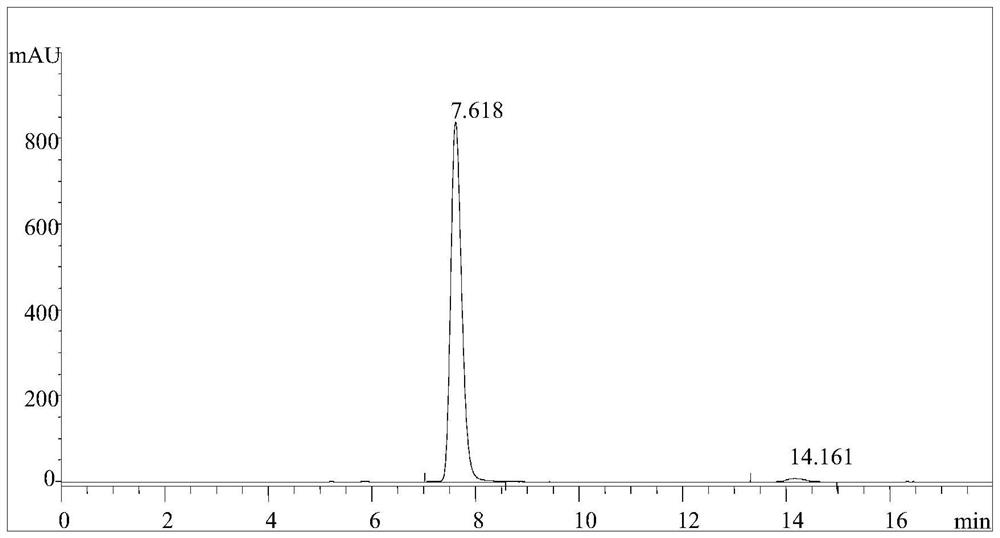
Carbonyl Reductase Mutant and Its Application in Catalytic Synthesis of 17β-Hydroxysteroids
A technology of hydroxysteroids and reductase, which is applied in the field of enzymes and enzyme engineering, can solve the problems of low conversion efficiency, low substrate concentration, and low conversion efficiency, and achieve simple and convenient preparation methods, less by-products, and short conversion time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Preparation of wild-type carbonyl reductase (RasADH) recombinant expression plasmid and recombinant expression transformant
[0032] After the sequence of SEQ ID No.1 was fully synthesized, it was connected to the pET21a vector, and then transformed into E. coli BL21(DE3) competent cells, and positive clones were picked to obtain the recombinant expression transformant E.coli BL21(DE3) / pET-21a-RasADH.
Embodiment 2
[0033] Embodiment 2: Carbonyl reductase RasADH mutant construction
[0034] In order to improve the activity of wild-type carbonyl reductase RasADH (its amino acid sequence is SEQ ID No.1, and its coding nucleotide sequence is SEQ ID No.3), site-directed saturation mutation was carried out at position 205, and the specific steps are as follows:
[0035] (i) Introduce mutations: design forward and reverse primers according to the nucleotides shown in SEQ ID No.3, and the specific primers are shown in Table 1 below:
[0036] Table 1
[0037] site upstream primer downstream primer F205A CTGCGTGCTAAAGCTGCTGCTGCTACC GGTAGCAGCAGCAGCAGCTTTAGCACGCAG F205R CTGCGTGCTAAAAGGGCTGCTGCTACC GGTAGCAGCAGCCTTTTAGCACGCAG F205K CTGCGTGCTAAAAAGGCTGCTGCTACC GGTAGCAGCAGCCTTTTTAGCACGCAG F205I CTGCGTGCTAAAATTGCTGCTGCTACC GGTAGCAGCAGCAATTTTAGCACGCAG F205H CTGCGTGCTAAACATGCTGCTGCTACC GGTAGCAGCAGCATGTTTAGCACGCAG F205L CTGCGTGCTAAACTTGCTGCTG...
Embodiment 3
[0053] Example 3: Construction of recombinant cells co-expressed with carbonyl reductase RasADH mutant and glucose dehydrogenase (GDH)
[0054] Using the RasADH mutant gene constructed in Example 2 and the existing glucose dehydrogenase gene in the laboratory as templates, these mutant genes and GDH genes were amplified by means of PCR, and after purification, they were respectively connected to the co-expression vector pRSFDuet. The two reading frames were successfully verified and transformed into Escherichia coli competent BL21 (DE3), and positive clones were picked to obtain recombinant cells co-expressing the RasADH mutant and GDH.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com