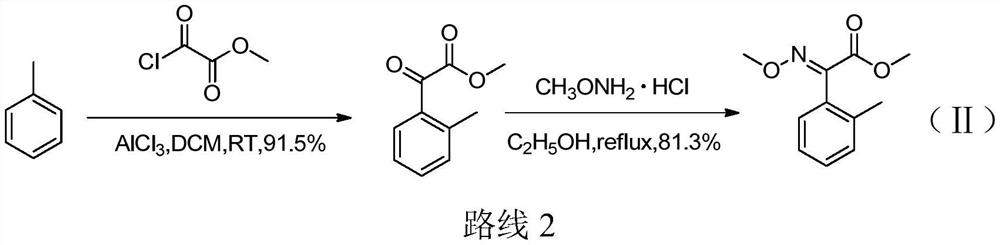
Preparation method of (E)-2-methyl-alpha-methoxyimino methyl phenylacetate and intermediate thereof
A technology of hydroxyliminophenylacetonitrile and hydroxyliminobenzene, which is applied in the field of preparation of methyl-2-methyl-α-methoxyiminophenylacetate and its intermediates, can solve the problem of harsh operating conditions, A lot of waste water, high production cost and other problems, to achieve the effect of mild reaction conditions, shorten the reaction steps, and reduce the preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] In the present embodiment, 2-methyl-alpha-hydroxyiminophenylacetic acid is synthesized, and its method is as follows:
[0060] 88.9g (0.43mol) of 2-methyl-α-hydroxyiminophenylacetonitrile, add 150g of water, 21.5g of sodium hydroxide (0.52mol, content 96%), heat up to reflux reaction for 4 hours, cool down to about 10°C 110.6g (mass fraction 37%) of concentrated hydrochloric acid was cooled to about 5°C, and the reaction solution was added dropwise, and after the addition was completed, stirring was continued at 5-10°C for 1 hour, filtered, and the filter cake was rinsed with cold water and dried to obtain 75.6 g2-Methyl-α-hydroxyiminophenylacetic acid (the product is a mixture of Z configuration and E configuration, according to the peak areas of the two isomers in high performance liquid chromatography, the Z / E ratio is 5 / 94) , HPLC quantitative analysis, content 98.8%, yield 97.0%, melting point: 137-139°C. 1 H-NMR (D 6 -DMSO), δ:2.26(s, 3H, Ar-CH 3 ), 7.19-7.41 (...
Embodiment 2
[0062] In the present embodiment, 2-methyl-alpha-hydroxyiminophenylacetic acid is synthesized, and its method is as follows:
[0063] 88.9g (0.43mol) 2-methyl-α-hydroxyiminophenylacetonitrile, add 150g of water, 33.0g (0.86mol, content 96%) of sodium hydroxide, heat up to 60°C for 24 hours, then cool down to 10°C About; 149.3g of concentrated hydrochloric acid (mass fraction 37%) was cooled to about 5°C, added dropwise to the reaction solution, and after the addition was completed, continued to stir at 5-10°C for 1 hour, filtered, and the filter cake was rinsed with cold water and dried to obtain 74.6g 2-methyl-α-hydroxyiminophenylacetic acid (the product is a mixture of Z configuration and E configuration, Z / E ratio 3 / 96), HPLC quantitative analysis, content 99.0%, yield 96.0%
Embodiment 3
[0065] In the present embodiment, 2-methyl-alpha-hydroxyiminophenylacetic acid is synthesized, and its method is as follows:
[0066] 88.9g (0.43mol) 2-methyl-α-hydroxyiminophenylacetonitrile, add water 150g, sodium hydroxide 15.0g (0.39mol, content 96%), heat up to reflux reaction for 8 hours, cool down to about 10°C 110.6g (mass fraction 37%) of concentrated hydrochloric acid was cooled to about 5°C, and the reaction solution was added dropwise, and after the addition was completed, stirring was continued at 5-10°C for 1 hour, filtered, and the filter cake was rinsed with cold water and dried to obtain 73.9 g 2-methyl-α-hydroxyiminobenzene (Z / E mixture, Z / E ratio 4 / 95), HPLC quantitative analysis, content 98.9%, yield 95.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com