Anti-cd137 antigen-binding molecule and utilization thereof
An antigen-binding molecule, CD137 technology, applied in applications, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, antibody medical components, etc., can solve problems such as damage to normal cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1130] Example 1: Antigen Preparation
[1131] (1-1) Preparation of human CD137 extracellular domain
[1132] The extracellular domain of human CD137 (also known as hCD137) was prepared using methods known to those skilled in the art. Specifically, downstream of the gene fragment encoding the extracellular region of human CD137, a gene fragment encoding a histidine tag and a gene fragment encoding a biotin-added specific sequence (AviTag sequence, SEQ ID NO: 86) were connected. A gene fragment encoding a protein (human CD137 or hCD137-HisBAP, SEQ ID NO: 87) in which the extracellular region of human CD137, histidine tag and Avitag are linked was incorporated into an animal cell expression vector. The constructed plasmid vector was transfected into FreeStyle293 cells (Invitrogen) using 293-fectin (Invitrogen). At this time, a plasmid vector containing a gene expressing EBNA1 (SEQ ID NO: 88) was simultaneously transfected. Place the cells transfected with the gene accordi...
Embodiment 2
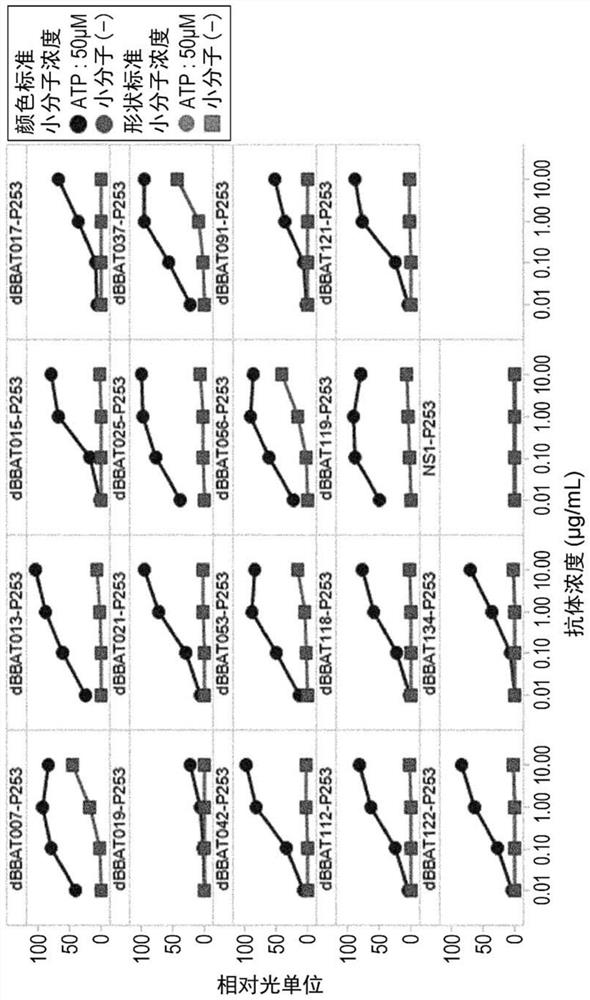
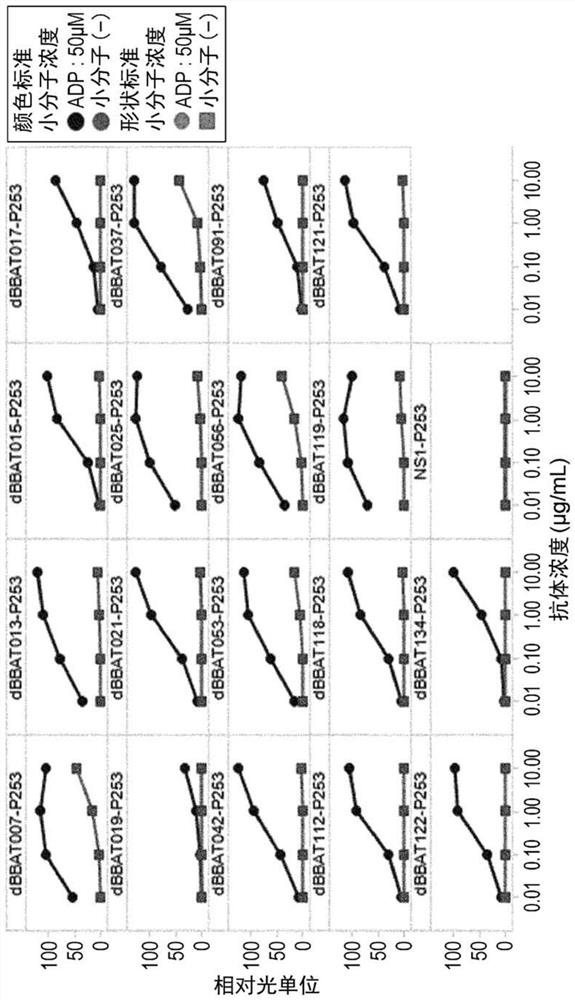
[1147] Example 2: Obtaining ATP-dependent CD137 antibody
[1148] (2-1) Using ATP to obtain antibodies with small molecule-dependent antigen-binding activity through rationally designed libraries (Small Molecule Conversion Antibody)(1)
[1149] (2-1-1) Panning
[1150] Antibodies exhibiting binding activity to antigen in the presence of adenosine triphosphate (adenosine 5'-triphosphate; ATP) were obtained from a rationally designed antibody phage display library constructed in previous patent WO2015 / 083764. Note that an antibody with small molecule-dependent antigen (eg, CD137) binding activity may be referred to as a "switch antibody" or "small molecule switch antibody", and an antibody with ATP-dependent antigen (eg, CD137) binding activity is referred to as "Converted antibody" or "ATP-converted antibody". To obtain, phages presenting antibodies that exhibited binding activity to antigen captured on beads in the presence of ATP were harvested. Subsequently, phage...
Embodiment 3
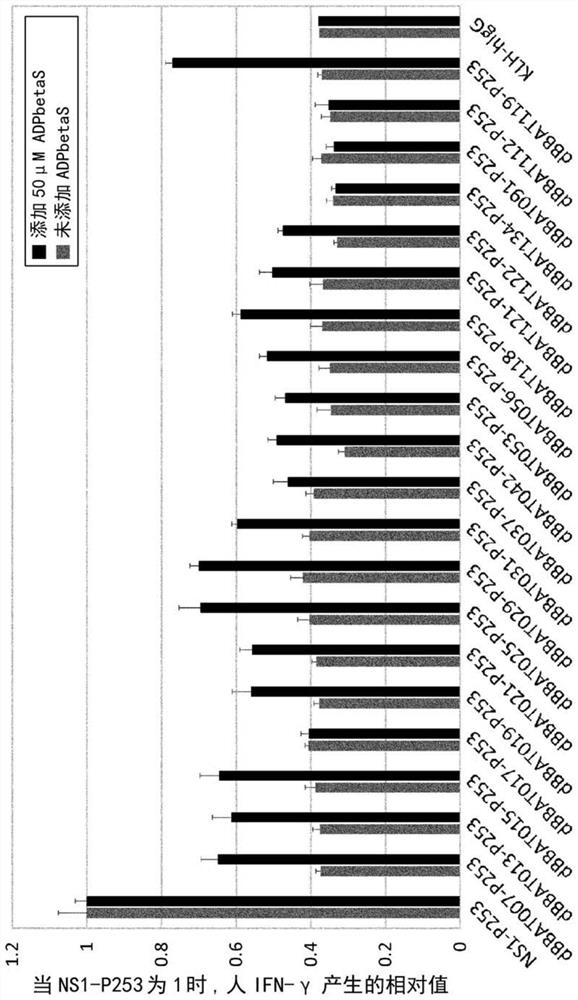
[1217] Example 3: Enhancement of Antigen Binding Antibodies in the Presence of Small Molecules Using Rationally Designed Light and Heavy Chain Libraries binding activity
[1218] (3-1) Construction of a library for enhancing binding activity using a rationally designed light chain library
[1219] For the antibody library containing a large number of antibodies having ATP-dependent antigen-binding activity harvested in Example 2-2-1, the binding activity was enhanced by reconstituting the antibody light chain library.
[1220] The light chain and heavy chain regions of a rationally designed antibody phage display library constructed in the existing patent WO2015 / 083764 were used to construct an antibody light chain library and an antibody heavy chain library to enhance binding activity. They were introduced into the light chain or heavy chain regions of the above-mentioned light chain library or the phagemid vector library harvested in Example 2-2-1, and introduced into ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com