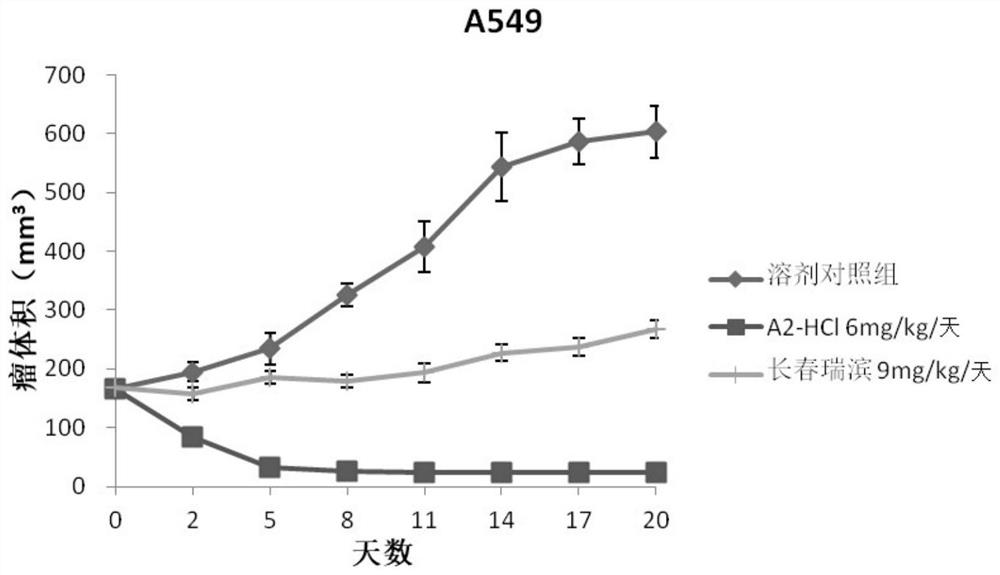
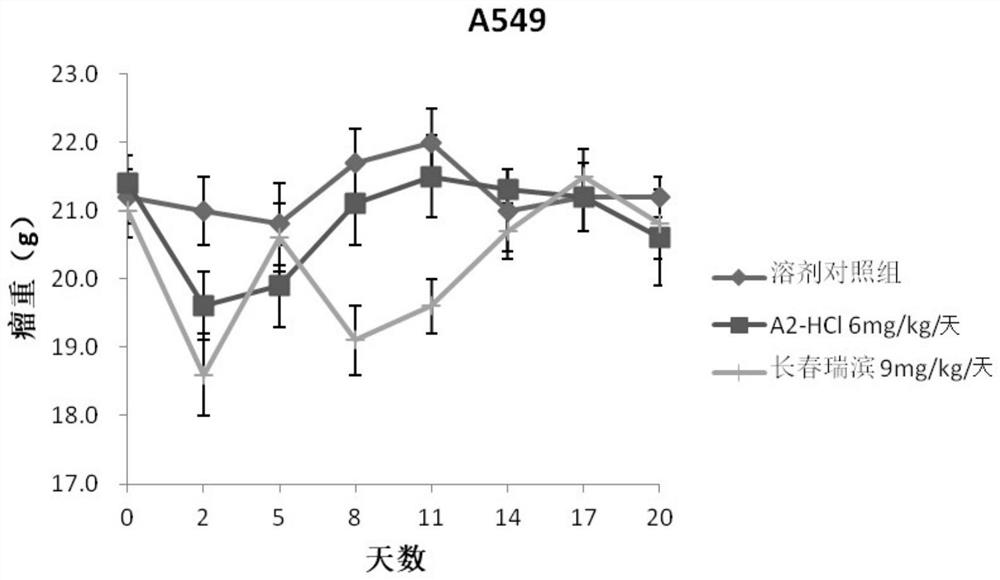
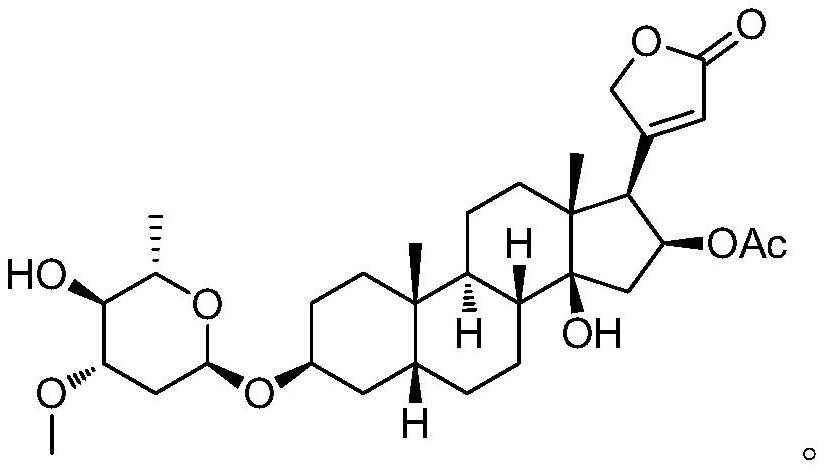
Oleandrin derivative and preparation method, pharmaceutical composition and use thereof
A technology of oleandrin and its derivatives, which is applied in the field of medicinal chemistry, can solve the problems of unsatisfactory physical and chemical properties, low water solubility of oleandrin, and poor drug-like properties, and achieve abundant synthetic raw materials, simple preparation methods, The effect of improving water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1: Synthesis of Compound A1
[0058]
[0059] Add oleandrin (0.3mmol, 172mg), N-acetylglycine (0.6mmol, 70mg, 2.0eq), DMAP (0.3mmol, 37mg, 1.0eq), HATU (0.6mmol, 228mg, 2.0eq) to the reaction flask eq) was dissolved in dichloromethane (5mL), triethylamine (0.9mmol, 125μL, 3.0eq) was added, then EDCI (0.9mmol, 172mg, 3.0eq) was slowly added, and the reaction was stirred at room temperature for 6h. After the reaction was completed, dichloromethane was added to dilute, and the reaction solution was diluted with saturated Na 2 CO 3 The solution was washed twice, the saturated sodium chloride solution was washed twice, the dichloromethane layer was dried over anhydrous sodium sulfate, concentrated under reduced pressure and subjected to column chromatography (7:1~3:1, petroleum ether / acetone) to obtain the target product, Yield 56%.
[0060] 1 H NMR (500MHz, DMSO-d 6 )δ5.96(s,1H),5.37(s,1H),4.98–4.87(m,3H),4.47(s,1H),4.38(s,1H),3.82(s,1H),3.69(s ,1H),3.55(s,...
Embodiment 2
[0061] Example 2: Synthesis of Compound A2
[0062]
[0063] Add oleandrin (0.5mmol, 288mg, 1.0eq), N-Boc-L-valine (217mg, 1.0mmol, 2.0eq), DMAP (0.5mmol, 61mg, 1.0eq) to the reaction flask, HATU (1.0mmol, 380mg, 2.0eq, ) was dissolved in dichloromethane (5mL), triethylamine (1.5mmol, 207μL, 3.0eq) was added, and then EDCI (1.5mmol, 287mg, 3.0eq) was slowly added at room temperature The reaction was stirred for 2h. After the reaction was completed, dichloromethane was added to dilute, and the reaction solution was diluted with saturated Na 2 CO 3 The solution was washed twice, the saturated sodium chloride solution was washed twice, the dichloromethane layer was dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain the intermediate. The intermediate was dissolved in acetone (5 ml), trifluoroacetic acid (TFA) (2.6 mL) was added, and the mixture was stirred at room temperature for 48 h. After the reaction was completed, dichloromethane was...
Embodiment 3
[0065] Example 3: Synthesis of Compound A3
[0066]
[0067] The reaction was performed as in the preparation of A2, except that the starting material used N-Boc-L-alanine instead of N-Boc-L-valine. The target product A3 was obtained after column chromatography (100:1:0.5~100:2:0.5, dichloromethane / methanol / triethylamine) with a yield of 46%.
[0068] 1 H NMR (CDCl 3 ,400MHz)δ5.95(s,1H),5.46(t,J=9.2Hz,1H),5.01–4.82(m,3H),4.65(t,J=9.5Hz,1H),3.88(s,1H ), 3.83–3.76 (m, 1H), 3.66–3.60 (m, 1H), 3.58–3.3 (m, 1H), 3.31–3.06 (s, 3H), 3.18 (d, J=8.6Hz, 1H), 2.72(dd, J=15.4, 9.8Hz, 1H), 2.21(dd, J=12.6, 3.8Hz, 1H), 1.95(s, 3H), 1.89–1.59(m, 11H), 1.63–1.42(m, 6H), 1.37–1.23(m, 3H), 1.35(d, J=7.0Hz, 3H), 1.11(d, J=5.7Hz, 3H), 0.94(s, 3H), 0.92(s, 3H); ESI-MS(m / z)648[M+1] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com