Chiral benzodihydrothiapyran-4-one compound as well as preparation method and application thereof
A technology for chroman and ketone compounds, which is applied in the field of chiral compound synthesis, can solve problems such as unreported synthesis, and achieve the effects of less photocatalyst dosage, mild reaction conditions, and less metal-free participation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] ( R )-2-(2-(2′-pyridine) ethyl) chroman-4-ketone III-1 The specific preparation steps are as follows:
[0034]
[0035] Preparation procedure: Take a dry 25 mL schlenk tube and add 24.3 mg (0.15 mmol) of 4H -Benzothiopyran-4-one I-1, 10.5 mg (0.1 mmol) vinylpyridine II-1, DPZ (0.7 mg, 0.002 mmol), chiral phosphoric acid catalyst BA (13.3 mg, 0.02 mmol), Hans Esters HE 55.6 mg (0.18 mmol), then add 1 mL of dichloromethane, cover the bottle cap, degas with a vacuum pump 2-3 times at no higher than -78°C, 5-10 min each time, and inject Protected by argon, then placed at -25°C, after standing still for half an hour, irradiated with a 3 W blue light with a wavelength of 410-510 nm at a distance of about 3 cm from the schlenk tube, and reacted for 60 hours. After the reaction, the column layer Analysis and separation (petroleum ether / ethyl acetate = 10~3:1, volume ratio), concentration by rotary evaporation, and vacuum drying (drying at 25°C for 1 hour) yielded 23.4 mg of...
Embodiment 2
[0042] ( R )-2-(2-(2´-pyridine) ethyl)-6-fluorochroman-4-one III-2 The specific preparation steps are as follows:
[0043]
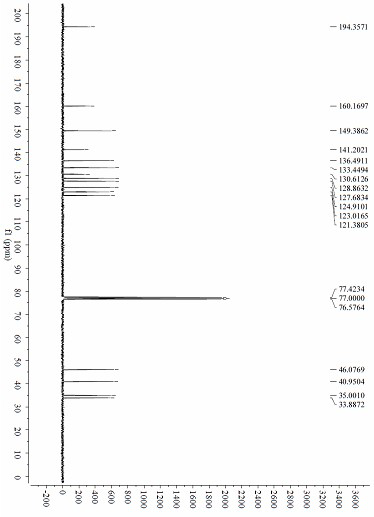
[0044] In this embodiment, the 4 in embodiment 1 H -benzothiopyran-4-one I-1 is replaced with 6-fluorobenzothiopyran-4-one I-2, and other steps are the same as in Example 1 to obtain 20.7 mg yellow oil ( R )-2-(2-(2´-pyridine) ethyl)-6-fluorochroman-4-one III-2, yield 72%, enantiomeric excess 94%. NMR and mass spectrometry data are: 1 H NMR (300 MHz, CDCl 3 ) δ 8.51 (d, J = 4.3 Hz, 1H), 8.02 (d, J = 2.0 Hz, 1H), 7.59 (t, J = 7.6Hz, 1H), 7.33 (dd, J = 8.4, 2.1 Hz, 1H), 7.21 (d, J = 8.5 Hz, 1H), 7.13 (dd, J = 11.9, 7.5 Hz, 2H), 3.50 (dd, J = 10.6, 6.5 Hz, 1H), 3.04 (m, 2H), 2.87(m, 2H), 2.15 (m, 2H); 13 C NMR (75 MHz, CDCl 3 ) δ 193.24 (s, 1H), 159.96 (s,1H), 149.39 (s, 2H), 139.51 (s, 1H), 136.56 (s, 2H), 133.42 (s, 3H), δ 131.3(d, J = 34.1 Hz), 128.9 (d, J = 48.9 Hz), 123.0, 121.5, 45.7, 41.0, 34.9,33.7; HRMS (ESI) m / z 310.0672 (M+Na + ...
Embodiment 3
[0046] ( R )-2-(2-(2'-pyridine) ethyl)-8-bromochroman-4-one III-3 The specific preparation steps are as follows:
[0047]
[0048] In this embodiment, the 4 in embodiment 1 H -Bromothiopyran-4-ketone I-1 is replaced with 8-bromobenzothiopyran-4-ketone I-3, and other steps are the same as in Example 1 to obtain 18.0 mg yellow oil ( R )-2-(2-(2´-pyridine)ethyl)-8-bromochroman-4-one III-3, yield 52%, enantiomeric excess 98%. NMR and mass spectrometry data are: 1 H NMR (300 MHz, CDCl 3 ) δ 8.52 (d, J = 4.3 Hz, 1H), 8.05 (d, J = 7.9 Hz, 1H), 7.62 (m, 2H),7.14 (m, 2H), 7.04 (t, J = 7.8 Hz, 1H), 3.50 (dt, J = 14.0, 5.2 Hz, 1H), 2.93(m, 4H), 2.21 (dd, J = 14.8, 7.0 Hz, 2H); 13 C NMR (75 MHz, CDCl 3 ) δ 193.8,160.0, 149.4, 142.5, 137.3, 136.6, 132.4, 127.9, 125.2, 123.1, 121.6, 44.7,40.3, 34.8, 33.8; HRMS (ESI) m / z 348.0056 (M+H + ), calc. for C 16 h 15 NOSBr348.0052.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com