Penicillamine-based polypeptide disulfide bond synthesis method and application thereof
A synthesis method and penicillamine technology, applied in the field of chemical biology research, can solve problems such as difficulty in correct recombination of disulfide bonds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1 The synthesis of the polypeptide disulfide bond based on penicillamine
[0049] 1. Use WANG resin to synthesize resin A
[0050] Peptide solid-phase synthesis (taking WANG resin as an example): Weigh WANG resin into a peptide tube, add DCM, and blow nitrogen for 10 minutes to make it swell. Add 50% (v / v) morpholine in DMF and blow nitrogen gas for 30 minutes (twice) to remove the Fmoc protecting group. The resin was alternately washed 8 times with DMF and DCM, and the corresponding Fmoc-AA-OH (5eq), HCTU (5eq), and DIPEA (10eq) were dissolved in DMF and added to the resin for 1 hour under nitrogen blowing. Then alternately wash with DMF and DCM for 8 times, continue deprotection according to the above method, and connect another amino acid until the synthesis of the polypeptide sequence is completed.
[0051] 2. Synthesis and separation of polypeptides containing disulfide bonds (taking two main routes as examples):
[0052] Route 2: Take the resin (100m...
Embodiment 2
[0064] Example 2 Stability verification of polypeptides containing disulfide bonds in the presence of reducing agents:
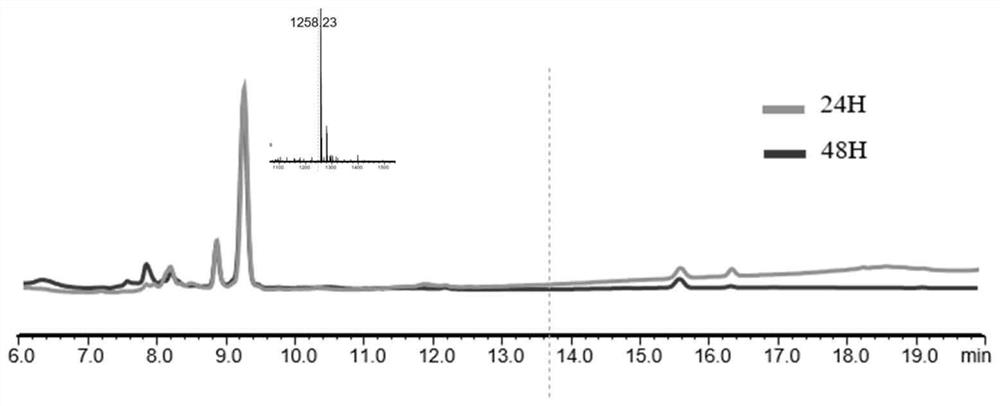
[0065] Dissolve the polypeptide (1mM) synthesized according to the method of Example 1 in 100mM PBS (phosphate buffer saline) with a pH value of 7.4, then add 10mM GSH or Pys, and incubate at 37°C in a water bath. The reaction solution after 24 hours and 48 hours was analyzed by liquid chromatography-mass spectrometry to observe the reduction of disulfide bonds.
[0066] The LCMS analysis pictures of the polypeptide Ac-Cyclo (PenSPC) NIYYKV-COOH (MW: 1257) at different times in the presence of GSH are as follows figure 1 , which are respectively identified by LCMS after co-incubating the polypeptide with GSH for 24 hours and 48 hours. It can be seen from the figure that the polypeptide has not been substantially reduced.
[0067] The LCMS analysis pictures of the polypeptide Ac-Cyclo (PenSPC) NIYYKV-COOH (MW: 1257) at different times in the presence of Pys...
Embodiment 3
[0069] Example 3 Stability verification of polypeptides containing disulfide bonds in the presence of alkylating reagents (taking 1,3-2 bromomethylbenzene as an example):
[0070] The polypeptide synthesized according to the method of Example 1 was dissolved in 100 mM PBS with a pH value of 7.4, and the alkylating agent (1.5 mM) dissolved in DMSO (2-methyl sulfoxide) was added, and reacted in the presence of 1% formic acid for 24 hours, followed by analysis by liquid chromatography-mass spectrometry to observe the changes in the reaction system.
[0071] The LCMS analysis diagram of the polypeptide Ac-cycle (CSPAPen) IYYKV-COOH (MW: 1215) reacted for 24 hours in the presence of an alkylating reagent is shown in Figure 4 , that is, the peptide and the alkylating agent 1,3-dibromomethylbenzene were reacted for 24 hours in the presence of 1% formic acid, and then analyzed by LCMS. It can be seen from the figure that the polypeptide is basically unchanged.
[0072] The LCMS ana...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com