Preparation method of benidipine hydrochloride
A technology of benidipine hydrochloride and concentrated sulfuric acid, applied in organic chemistry and other directions, can solve the problems of complicated operation, high operation risk, long reaction time, etc., and achieves mild reaction process conditions, simple operation and post-processing, and short reaction steps. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048]Embodiment 1: the preparation of intermediate 2-(3-nitrobenzylidene)-methyl acetoacetate (Ⅰ)
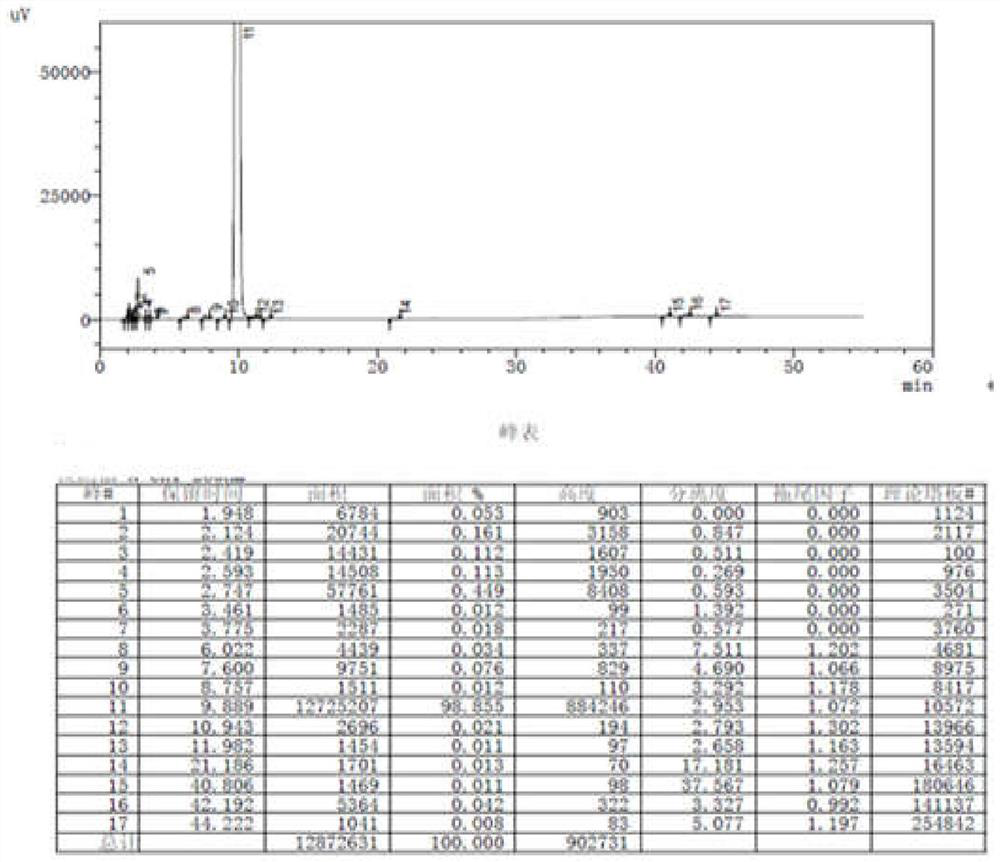
[0049] Add 34.8g (0.3mol) of methyl acetoacetate and 5.0ml of glacial acetic acid into the reaction flask, then slowly add 5.0ml of concentrated sulfuric acid at 0-10°C, stir for 15 minutes, then slowly add 30.2g (0.2mol) of 3-nitrobenzaldehyde, the temperature of the mixture was controlled at 20-30°C and the reaction was stirred for 1-2 hours, then 20ml of absolute ethanol was added, and stirred for 30 minutes, a large amount of solid crystals appeared, filtered, and the filter cake was used in 10ml of anhydrous After washing with water and ethanol, the solid was air-dried at 60-70° C. for 8 hours to obtain 43.0 g of a white solid, with a yield of 86.3%. HPLC purity (area normalization method): 98.855%. For HPLC results, see figure 1 .
Embodiment 2
[0050] Embodiment 2: the preparation of intermediate 2-(3-nitrobenzylidene)-methyl acetoacetate (Ⅰ)
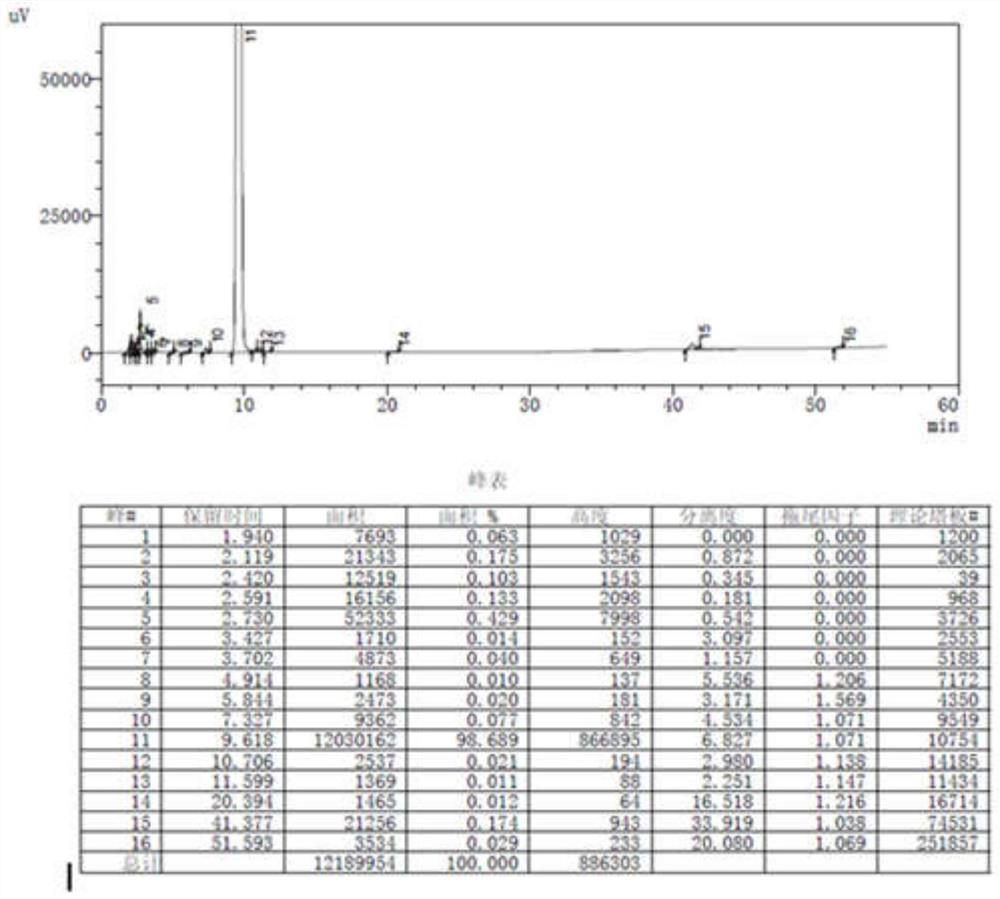
[0051] Add 34.8g (0.3mol) of methyl acetoacetate and 5.0ml of glacial acetic acid into the reaction flask, then slowly add 5.0ml of piperidine at 0-10°C, stir for 15 minutes, then slowly add at 0-10°C 30.2g (0.2mol) of 3-nitrobenzaldehyde, the temperature of the mixture was controlled at 20-30°C and the reaction was stirred for 1-2 hours, then 20ml of absolute ethanol was added, and stirred for 30 minutes, a large amount of solid crystals appeared, filtered, and the filter cake was used in 10ml of anhydrous After washing with water and ethanol, the solid was air-dried at 60-70° C. for 8 hours to obtain 41.0 g of a white solid, with a yield of 82.2%. HPLC purity (area normalization method): 98.689%. For HPLC results, see figure 2 .
Embodiment 3
[0052] Example 3: Preparation of intermediate 1,4-dihydro-2,6-dimethyl-4-(m-nitrophenyl)-3,5-pyridinedicarboxylate (II)
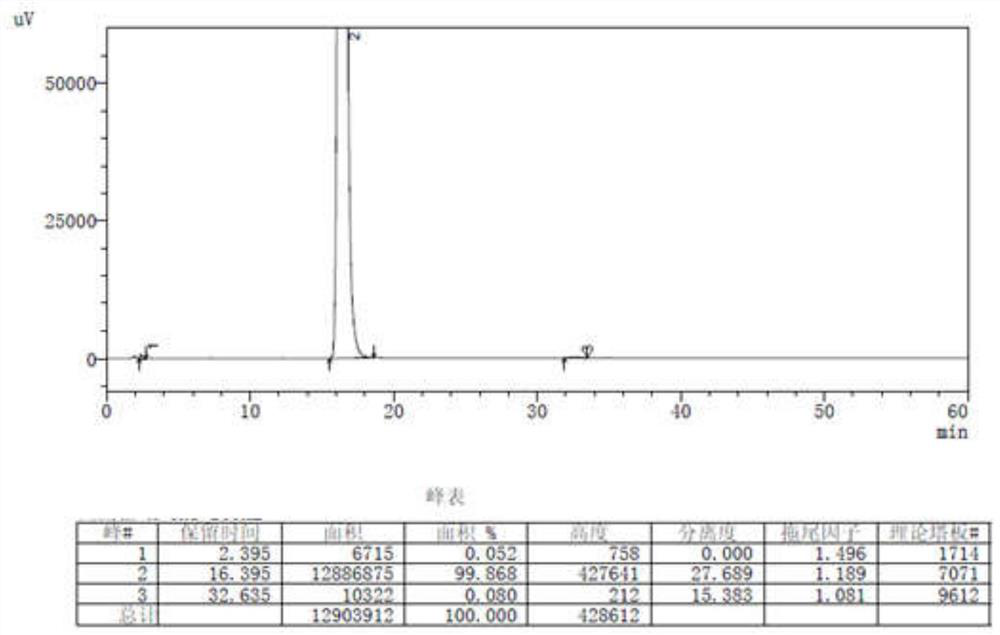
[0053] Add 20.0 g (0.08 mol) of 2-(3-nitrobenzylidene)-methyl acetoacetate, 11.5 g (0.1 mol) of methyl β-aminocrotonate and 150 ml of absolute ethanol in the reaction flask, and control the temperature of the mixture Stir and react at 40-50°C for 2-4 hours. After the reaction is complete, place in an ice-water bath at 0-10°C to cool and crystallize. After stirring for 1 hour, off-white crystals precipitated, filtered, and the solid was air-dried at 50-60°C for 8 hours to obtain 24.2 g of a light yellow solid, with a yield of 87.3%. HPLC purity (area normalization method): 99.868%, HPLC see results image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com