Xylanase GH11-xyllanase mutant and application of xylanase GH11-xyllanase mutant
A xylanase mutation and mutant technology, applied in the field of xylanase GH11-xylanase mutants, can solve problems such as high cost of enzymes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Determination of key amino acid residues in the active framework of xylanase GH11-xylanase
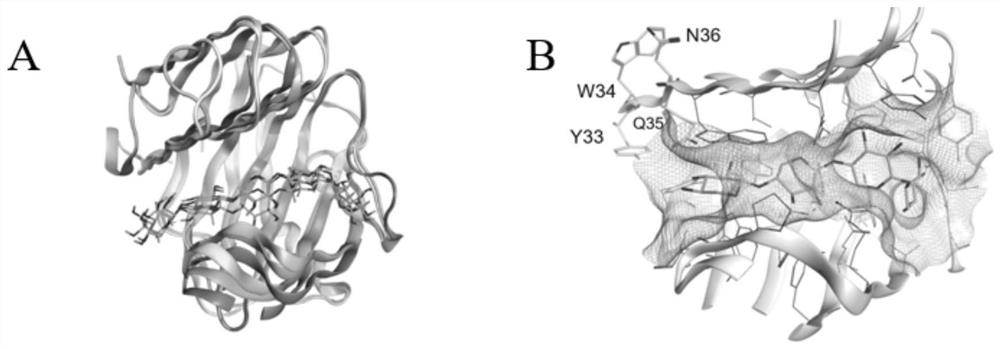
[0031] The xylanase GH11-xylanase selected in the present invention is a typical endo-degrading enzyme, and the final products of its hydrolysis of xylan are mainly xylobiose and xylotriose. Studies have shown that xylanases from different sources have different substrate specificities and can produce xylooligosaccharides with different degrees of polymerization. The present invention found that the xylanase BsCel5A derived from B. subtilis 168 has the highest sequence similarity (99%) with the xylanase GH11-xylanase through BLAST search, and obtained the crystal of xylanase BsCel5A in the PDB database structure (PDB ID: 3PZT), using it as a template, the three-dimensional structure model of xylanase GH11-xylanase (SEQ ID No: 1) was constructed ( figure 1 A). Then, through self-established screening principles, combined with PDB and CAZy database analysis, and compre...
Embodiment 2
[0033] Example 2 Construction of xylanase GH11-xylanase mutant library
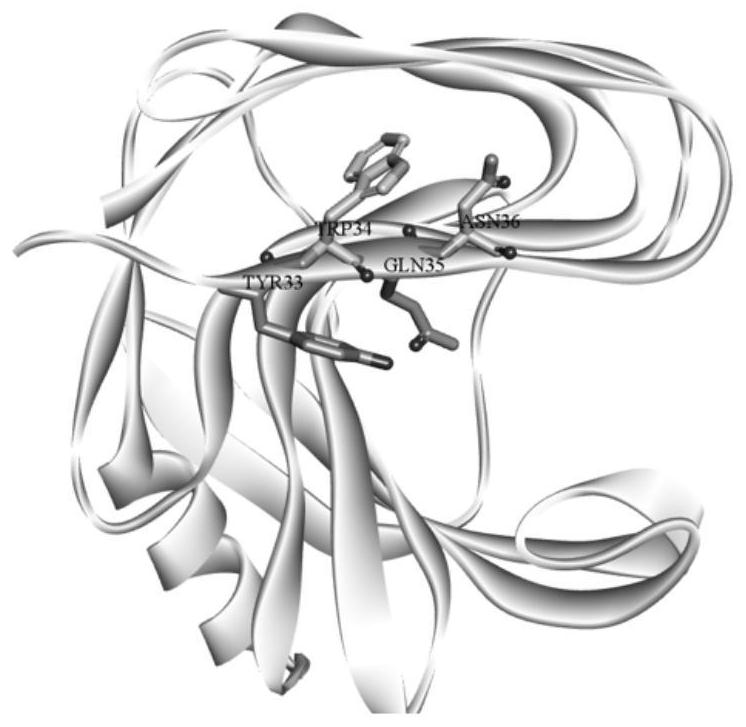
[0034] Analyze the relative position of SEQ ID No: 1 sequence 33-36 hotspot amino acid residues (see figure 2 ), it was found that Y33 and Q35 are closer, and the side chain substitutions of the two may have a greater impact on each other, so Y33 and Q35 were subjected to iterative saturation mutation (see image 3 ), saturation mutations were performed on W34 and N36, which were relatively far away.
[0035] Taking the W34 site as an example, the construction method of its mutant library is as follows, and the primers used are shown in the following table:
[0036] Primer Sequence(5' to 3') W34C-F GGCGCTAGCACAGACTACTGCCAAAATTGGACTGATG SEQ ID NO: 4 W34D-F GGCGCTAGCACAGACTACGACCAAAATTGGACTGATG SEQ ID NO: 5 W34E-F GGCGCTAGCACAGACTACGAACAAAATTGGACTGATG SEQ ID NO: 6 W34F-F GGCGCTAGCACAGACTACTTTCAAAATTGGACTGATG SEQ ID NO: 7 W34G-F GGCGCTAGCACAGACTACGGTCA...
Embodiment 3
[0038] Fermentation of embodiment 3 recombinant xylanase mutants in Escherichia coli
[0039] Each of the W34 mutants constructed in Example 2 and the original enzyme GH11-xylanase were inoculated into 50 mL of LB liquid medium containing 100 μg / mL kanamycin sulfate, and cultured overnight at 37° C. at 180 rpm; at an inoculum size of 2% Inoculate the seed solution into fresh 50mL LB liquid medium, cultivate to OD at 37°C and 180rpm 600 When it is 0.6-1.0, take it out and cool it in an ice-water bath for 5 minutes, add the inducer IPTG (isopropyl-β-D thiogalactopyranoside) (final concentration 0.1mmol / L), induce expression at 20°C, 150rpm for 20h.
[0040] Get the fermented liquid of induced expression, centrifuge at 12000rpm for 20min, discard the supernatant, then wash with 50mM Na 2 HPO 4 -KH 2 PO 4 (pH 7.0) buffer to resuspend and wash the bacterial cells, centrifuge at 12000rpm for 20min, discard the supernatant, resuspend with buffer, and then sonicate. The broken li...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com